

Less is more for health and happiness
- …


Less is more for health and happiness
- …


What Is Thyroid Eye Disease?
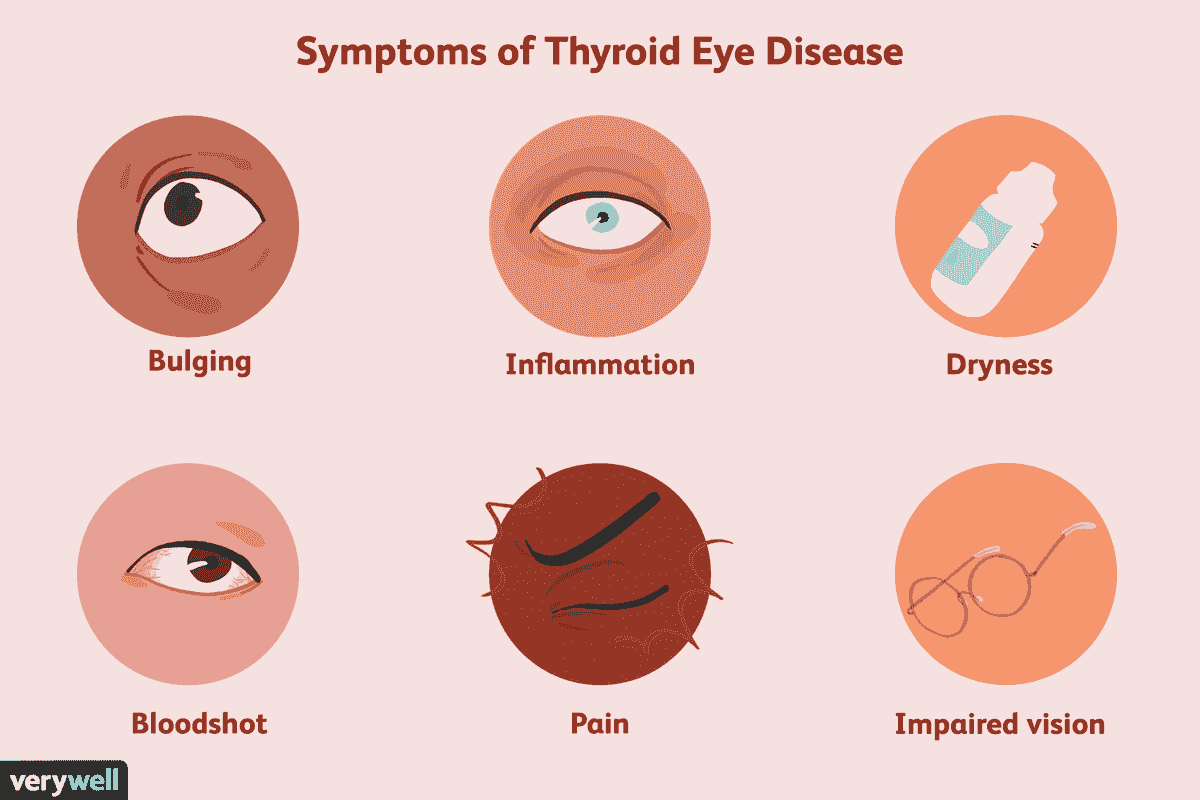
TED stands for Thyroid Eye Disease, which is an autoimmune condition often associated with Graves' disease. It affects the tissues around the eyes, causing inflammation, swelling, and sometimes symptoms like bulging eyes, double vision, and discomfort. TED can significantly impact a person's quality of life, both physically and emotionally.
It's important to recognize TED early and manage it with a multidisciplinary approach, including endocrinologists and ophthalmologists. Achieving normal thyroid levels (euthyroidism) is a key part of treatment, and guidelines like those from EUGOGO help tailor care based on the severity and activity of the disease.
Symptoms
- Bulging eyes (proptosis)
- Eye irritation
- Swollen and inflamed eyelids (blepharitis)
- Dry eyes or teary eyes
- Frequent blinking
- Light sensitivity (photophobia)
- Eye pain and headaches
- Difficulty moving your eyes
- Double vision (diplopia)
Causes/Risk factors
- Autoimmune diseases
- Graves' diseases
- TSHR stimulating autoantibodies
- Abnormal thyroid hormone levels
- Radioiodine therapy
- Smoke
- Characteristics of high-risk thyroid eye disease patients
Managment
- Eye drops
- Selenium supplements
- Eyeglasses with prisms
- Thionamides
- Corticosteroids
- Teprotumumab
- Radiation therapy
- Clinical trials
5 Effective Ways to Reduce Stress
Thoughts, musings, and ruminations
March 1, 2026 · science,artRead more...The sun shone brightly over the seaside dock, and a gentle breeze carried the faint scent of...February 7, 2026 · scienceRead more...1. Eyes "bulging out" – The eyeball protrudes from its socket, causing the thyroid gland to...January 31, 2026 · scienceRead more...I. First, a review: What are autoantibodies against TSH receptors? Remember in Graves'...January 24, 2026 · scienceRead more...I. First, let's understand: What is Graves' disease? Our bodies have an "immune system...January 17, 2026 · scienceRead more...Let's use the analogy of the thyroid hormone factory again, explaining the relationship between...January 10, 2026 · scienceRead more...1. Thyroid-stimulatinghormone (TSH): The "messenger" of the thyroid gland. It resides in a small...January 3, 2026 · scienceRead more...Thyroid eye disease is an autoimmune disorderclosely related to Graves' disease. It causes...December 12, 2025 · clinical trials,scienceRead more...Background 1.1 Thyroid Eye Disease (TED) Thyroid eye disease (TED) is a disabling condition...October 30, 2025 · mental healthRead more...Meaning in life, a global belief that one’s life has meaning and is significant, has long been...October 30, 2025 · mental healthRead more...Positive thinking is looking at the brighter side of situations, making a person constructive &...October 30, 2025 · mental healthRead more...Flourishing—a state of optimal mental health—has been linked to a host of benefits for the...Hello & Welcome
Add a subtitle here
Managing TED
What are nonsystemic treatment or lifestyle advices for TED?
Will lubricating eye drops be helpful?
What are the artificial tears?
What are the Fresnel press-on prisms?
Should patients stop smoking or avoid second-hand smoke exposure immediately?
Is ‘watchful monitoring’ strategy acceptable for moderate-to-severe TED patients?
What's the percentage of spontaneous disease inactivation and improvement?
What's the urgent treatment for sight-threatening TED?
What are the goals of the treatments for the active phase of TED?
What are the immunomodulatory treatments?
What's the mechanism of action of immunomodulatory therapy?
What are major adverse effects of immunomodulatory therapy?
Is immunomodulatory therapy efficient?
What's the referral guidance for patients with TED?
When should carry out surgical treatment for TED?
What's the medical therapy for mild TED?
What's the therapy to calm down orbital inflammation?
What's the second-line immunosuppressive agents?
Which type of surgery is recommended for mild TED?
What are available treatments for moderate-to-severe active TED ?
What's the risk factors for TED?
When are glucocorticoids used for TED?
What's the difference between OGC and IVGC?
What's the standarized dosing for GC?
What are adverse events (AEs) in relation to GC?
Which kind of patients are not suitable for GC treatment (Contraindications)?
What are adverse effects of medical therapy for thyroid eye disease?
How much is the drug cost for TED?
What are the impact of drug on vaccinations?
What are therapies for patients unresponsive or intolerant to GC?
What are the emergying therapies recently approved?
What are the emergying therapies in clinical trail stages?
How to find suitable clinical trial?
What outcome should patient expect for antcipating clinical trials?
Thyroid Eye Disease (TED)
What is Thyroid Eye Disease?
What's the common symptoms of TED?
What does double vision (diplopia) mean?
What do bulging eyes (proptosis) mean?
What does periorbital oedema and chemosis mean?
What does strabismus mean?
Who Should Get Tested for TED?
What's the difference between Graves’ Disease and Thyroid Eye disease?
What's the Tests for Thyroid Eye Disease ?
What's the Clinical Eye Exam of Thyroid Eye disease?
What's the Visual Acuity Test for Thyroid Eye disease?
What's the Exophthalmometry?
What's the Orbital Imaging (CT or MRI Scans)?
What's the Blood Tests for Thyroid Function?
How to Prepare for Testing of TED?
What Do the Results of TED Mean?
When to Retest or Monitor TED?
What does eye irritation or dryness mean?
What does excessive tearing or watery eyes mean?
Why is there redness or swelling around the eyes?
What is slit-lamp evaluation?
What are the assessment scores or systems for TED?
What does VISA (Vision, Inflammation, Strabismus, Appearance) mean?
What does Clinical Activity Score (CAS) mean?
What is GO-QOL (Graves’ Ophthalmopathy Quality of Life) questionnaire?
What is EUGOGO Classificationare of TED?
What is Modified NOSPECS Classification of TED?
Passion
Use a text section to describe your values, show more info, summarize a topic, or tell a story. Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore.
Independence
Use a text section to describe your values, show more info, summarize a topic, or tell a story. Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore.
Happiness
Use a text section to describe your values, show more info, summarize a topic, or tell a story. Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore.
Happiness
Use a text section to describe your values, show more info, summarize a topic, or tell a story. Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore.
FAQs
Add a subtitle here
What is the FAQ section?
What is Strikingly?
How do I create a website?
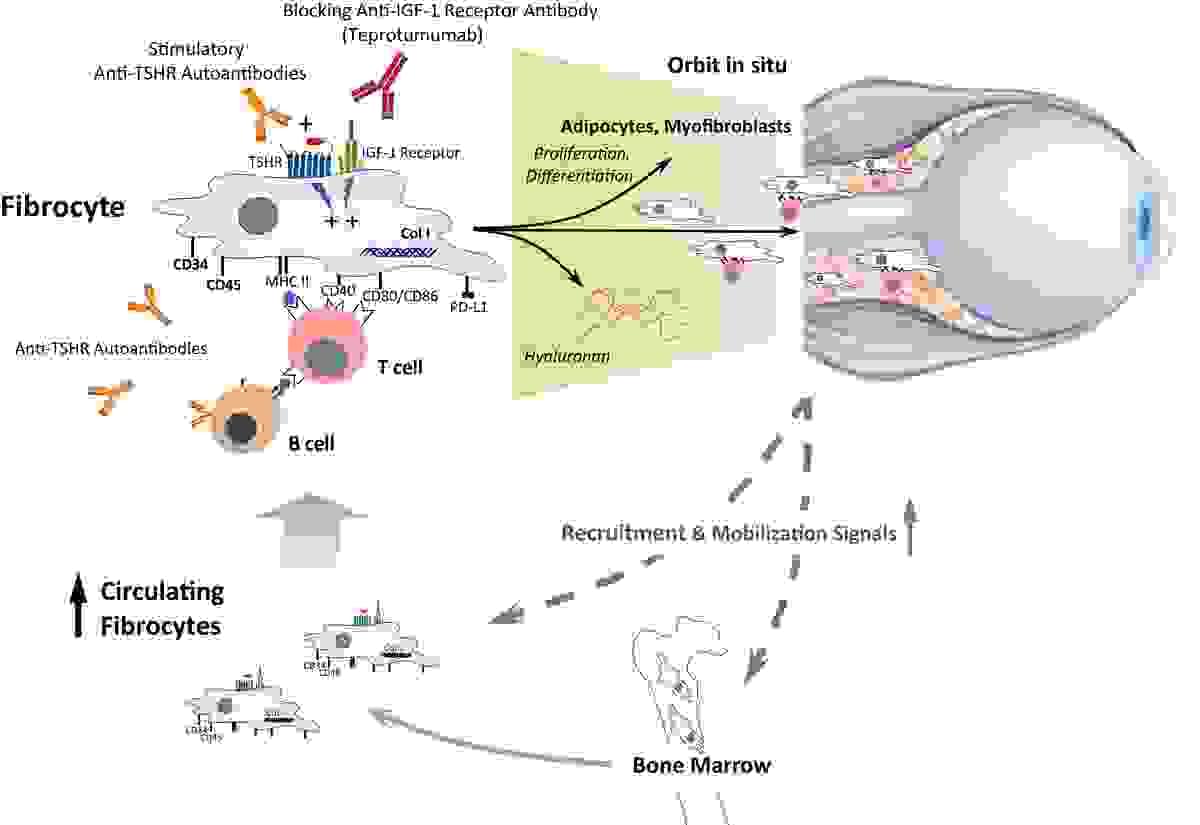
Thyroid eye disease is a complex inflammatory disorder
Thyroid Eye Disease (TED) is indeed a complex inflammatory disorder. It involves an autoimmune process where the body's immune system mistakenly targets tissues around the eyes. Research has shown that certain receptors, like the TSH receptor (TSHR) and the insulin-like growth factor-1 receptor (IGF-1R), are overexpressed on orbital fibroblasts in people with TED. Activation of these receptors leads to inflammation, tissue expansion, and changes such as swelling and bulging of the eyes.
This complex interaction of immune cells, antibodies, and receptors drives the characteristic symptoms of TED and makes its management challenging but also an exciting area for ongoing research and therapeutic advances.
FAQs
Add a subtitle here
What is the FAQ section?
What is Strikingly?
How do I create a website?
What Happens in My Eyes?
Let us understand our immune system first
Orbital fibroblasts
Human orbital fibroblasts are specialized cells found in the connective tissue of the orbit. They help maintain the structural integrity of the orbit, produce extracellular matrix components, and contribute to tissue repair and remodeling. They are also involved in various physiological and pathological processes, including inflammation and fibrosis.
TSHR
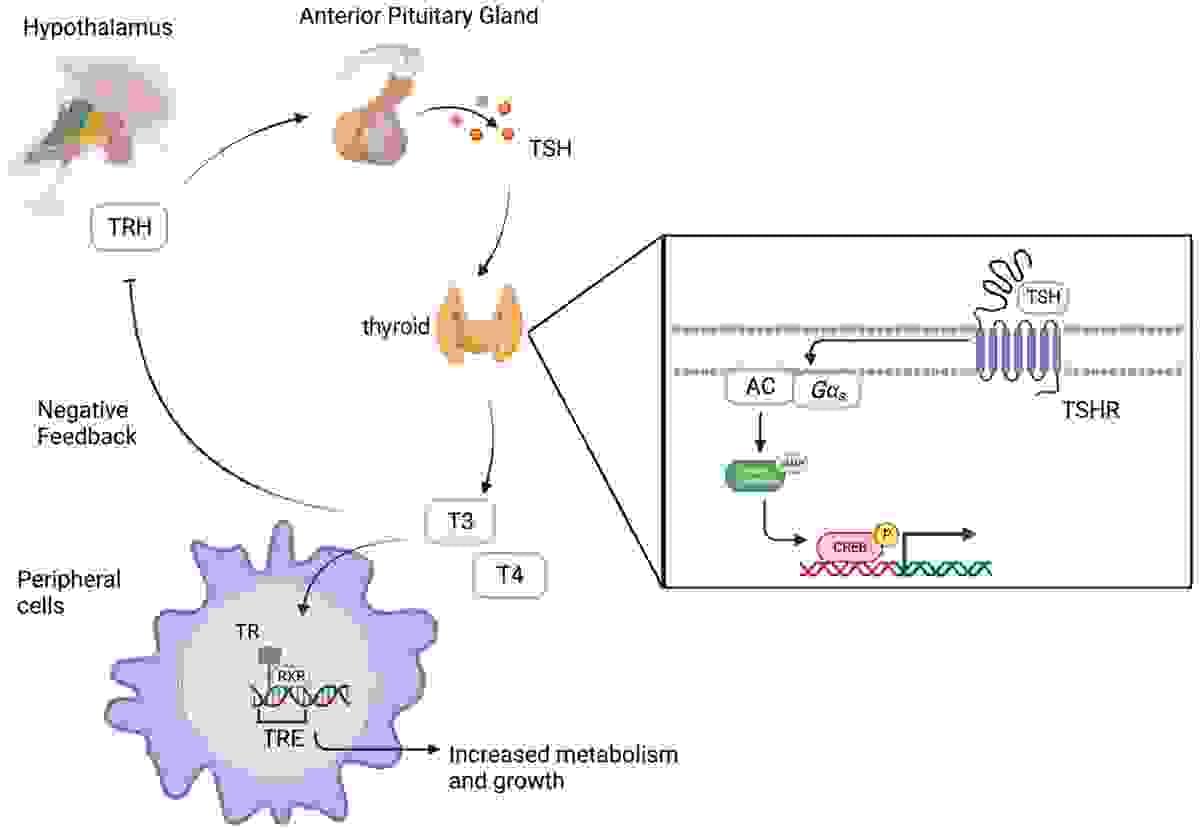
The thyrotropin receptor (or TSH receptor) is a receptor (and associated protein) that responds to thyroid-stimulating hormone (TSH, also known as "thyrotropin") and stimulates the production of thyroxine (T4) and triiodothyronine (T3). The TSH receptor is a member of the G protein-coupled receptor superfamily of integral membrane proteins and is coupled to the Gs protein.
Autoantibodies
Autoantibodies are a malfunctioning type of antibody.
Antibodies are proteins your immune system makes to identify and destroy invaders like germs, allergens or toxins in your blood. When your immune system detects a new unwanted substance in your body, it makes antibodies customized to find and destroy that invader.
Autoantibodies harm your body instead of keeping it healthy. They mistakenly target healthy tissue, instead of protecting you from substances that can make you sick. This damage can eventually cause many types of autoimmune diseases.
Thyroid-stimulating autoantibodies
Thyroid antibodies develop when a person’s immune system mistakenly attacks the thyroid cells and tissues. This leads to inflammation, tissue damage or disrupted thyroid function. These antibodies cause autoimmune thyroid disorders, such as Graves’ disease and Hashimoto’s thyroiditis.
If the initial thyroid test results show signs of a thyroid problem, and if there is a suspicion of autoimmune thyroid disease, one or more thyroid antibody tests may be ordered. Antibody tests are used to confirm the diagnosis of autoimmune thyroid diseases. Some people will test positive for more than one type of thyroid antibody.
In people with subclinical thyroid disease, the presence of antibodies can indicate the person may go on to develop full-blown thyroid disease in the future, but that treatment is not yet required. Positive antibodies can also be present in people without thyroid disease.
In Graves’ disease, the thyroid stimulating antibodies (TSAb) mimic the thyroid stimulating hormone (TSH) secreted by the pituitary gland. This causes the thyroid to continue to produce thyroid hormones, despite the pituitary trying to switch off the thyroid by stopping production of TSH. The presence of TRAb suggests a person has Graves’ disease. Approximately 95% of patients with Graves’ disease will have raised TRAb. The severity of Graves’ disease is often reflected in the levels of TRAb present. For example, where the TRAb levels are very high, the patient is less likely to achieve long-term remission following a course of treatment with antithyroid drugs.
It is sometimes possible for antibodies to be negative, but for a scan to confirm a Graves’ disease diagnosis.
IGF-1R
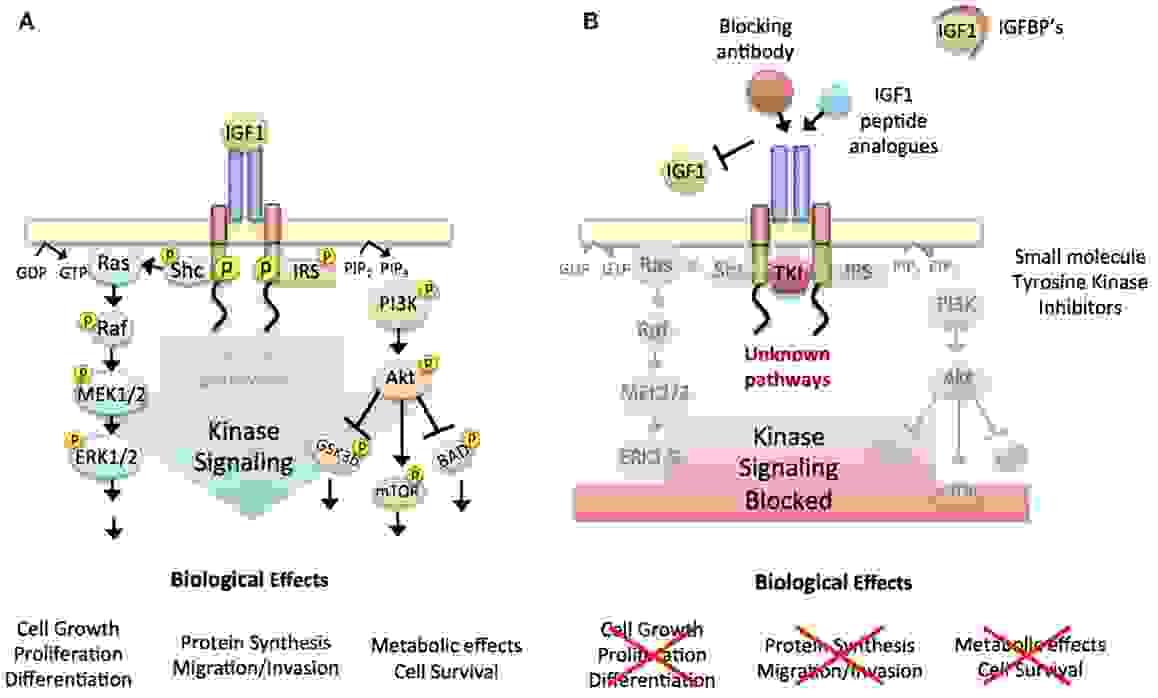
The insulin-like growth factor 1 (IGF-1) receptor is a protein found on the surface of human cells. It is a transmembrane receptor that is activated by a hormone called insulin-like growth factor 1 (IGF-1) and by a related hormone called IGF-2. It belongs to the large class of tyrosine kinase receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults – meaning that it can induce hypertrophy of skeletal muscle and other target tissues.
B cells
B cells are a type of white blood cell that makes infection-fighting proteins called antibodies. B cells are an important part of your immune system, your body’s defense against harmful pathogens (viruses, bacteria and parasites) that enter your body and make you sick.
B cells and T cells are a specific type of white blood cell called lymphocytes. Lymphocytes fight harmful invaders and abnormal cells, like cancer cells. T cells protect you by destroying pathogens and sending signals that help coordinate your immune system’s response to threats. B cells make antibodies in response to antigens (antibody generators). Antigens are markers that allow your immune system to identify substances in your body, including harmful ones like viruses and bacteria.
B cells are also called B lymphocytes.
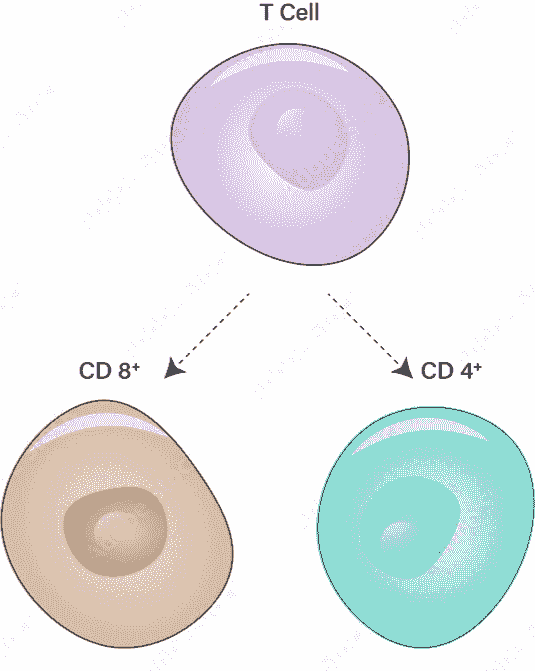
T cells
T cells are a type of white blood cell called lymphocytes. They’re also called T lymphocytes. Lymphocytes play an essential role in your immune system. Your immune system fights infection-causing pathogens (viruses, bacteria, fungi and parasites) and harmful cells, like cancer cells.
There are two main types of T cells:
Cytotoxic T cells: Cytotoxic T cells are also called CD8+ cells because they have a CD8 receptor on their membranes. These cells get their name from “cyto,” which means cell, and “toxic,” which means poisonous or harmful. Cytotoxic T cells kill cells infected with viruses and bacteria, and they also destroy tumor cells.
Helper T cells: Helper T cells are also called CD4+ cells because they have a CD4 receptor on their membranes. Unlike cytotoxic T cells, helper T cells don’t kill cells directly. Instead, they send signals that tell other cells in your immune system how to coordinate an attack against invaders. Helper T cells signal cytotoxic T cells, B cells and another type of white blood cell called a macrophage.
Although they’re not considered one of the main T cell types, regulatory T cells (suppressor cells) play an essential role in your immune system. These cells reduce the activity of other T cells when necessary. They can prevent T cells from attacking your body’s healthy cells.
MHC
The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.
Its name comes from its discovery during the study of transplanted tissue compatibility. Later studies revealed that tissue rejection due to incompatibility is only a facet of the full function of MHC molecules, which is to bind an antigen derived from self-proteins, or from pathogens, and bring the antigen presentation to the cell surface for recognition by the appropriate T-cells. MHC molecules mediate the interactions of leukocytes, also called white blood cells (WBCs), with other leukocytes or with body cells. The MHC determines donor compatibility for organ transplant, as well as one's susceptibility to autoimmune diseases.
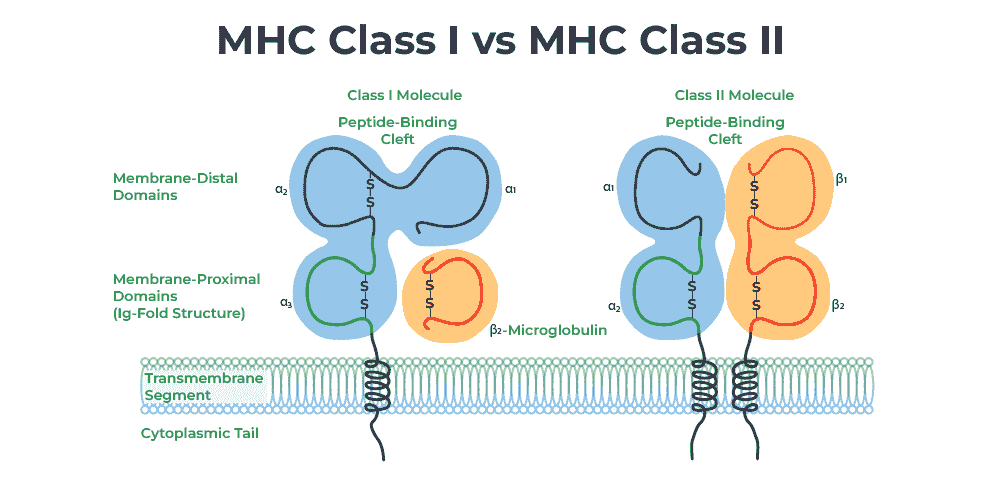
MHC II
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules normally found only on professional antigen-presenting cells such as dendritic cells, macrophages, some endothelial cells, thymic epithelial cells, and B cells. These cells are important in initiating immune responses.
MHC-II-expressing cells acquire antigen by distinct cellular processes that allow professional APCs to sample their external environment. Classically, extracellular proteins were thought to predominate as antigenic sources in MHC-II presentation, but many studies have demonstrated that the MHC-II peptidome largely consists of peptides derived from endogenous – rather than exogenous – source proteins.
Autoimmune diseases
Autoimmune diseases are health conditions that happen when your immune system attacks your body instead of defending it. Healthcare providers sometimes call them autoimmune disorders.
Usually, your immune system is like your body’s built-in security system. It automatically detects substances that shouldn’t be in your body (like viruses, bacteria or toxins) and sends out white blood cells to eliminate them before they can damage your body or make your sick.
If you have an autoimmune disease, your immune system is more active than it should be. Because there aren’t invaders to attack, your immune system turns on your body and damages healthy tissue.
Autoimmune diseases are chronic conditions. This means if you have an autoimmune disease, you’ll probably have to manage it and the symptoms it causes for the rest of your life.
Graves Disease
Graves’ disease is a lifelong (chronic) autoimmune disease that causes your thyroid to make too much thyroid hormone. It happens because your body makes antibodies to your thyroid gland.
It’s one of the most common causes of hyperthyroidism (overactive thyroid), especially if you have a family history of thyroid problems. Graves’ disease mainly affects your thyroid. But it can also affect your eyes and skin.
Graves’ disease speeds up your metabolism. This can affect several aspects of your health. You may not feel like yourself or even feel out of control of your body. It’s important to get medical treatment if you develop signs of this condition.
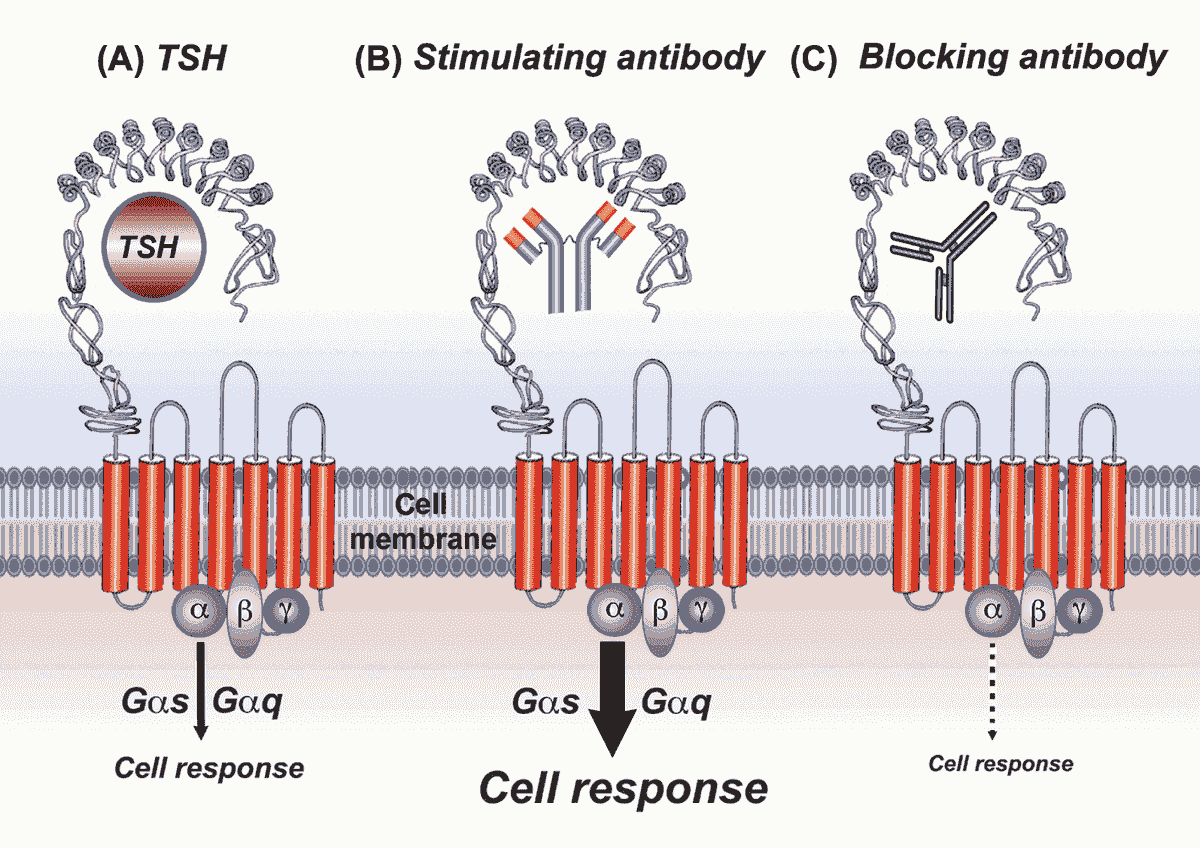
Graves’ disease (GD) is an autoantibody-mediated autoimmune disease that is characterized clinically by hyperthyroidism and pathologically by infiltration of thyroid by T and B cells reactive to the thyroid-stimulating hormone receptor (TSHR), thyroid peroxidase (TPO), and thyroglobulin (Tg) in most patients. GD is caused by direct stimulation of thyroid epithelial cells by TSHR stimulating antibodies (TSAb), triggering signaling cascades within thyrocytes that lead to over-production and secretion of thyroid hormones resulting in clinical hyperthyroidism .
The A subunit of the extracellular domain of TSHR is the major autoantigen in GD, mediating the T- and B-cell immune responses that cause GD. The sequence of events leading to GD start when pathogenic TSHR peptides are presented by HLA class II on antigen presenting cells to CD4+ T-cells. CD4+ T-cells then recognize the HLA class II-TSHR peptide complex and initiate immune responses, including signals for B-cell proliferation and differentiation into plasma cells, which produce and secrete anti-TSHR stimulating antibodies.
Thyroid eye disease (TED)
Graves’ disease (GD) is a common organ-specific autoimmune disease with an annual incidence of 20 to 50 cases per 100,000 persons. The primary symptoms are hyperthyroidism and goiter, with almost a half of GD patients reporting symptoms of Graves’ ophthalmopathy. Thyroid-stimulating hormone receptor (TSHR) is a major thyroid self-antigen, and autoantibodies against TSHR are widely believed to be the cause of hyperthyroidism. Autoantibodies that bind to specific epitopes on the TSHR mimic thyroid-stimulating hormone and induce the secretion of excessive amounts of thyroid hormone from thyroid cells.
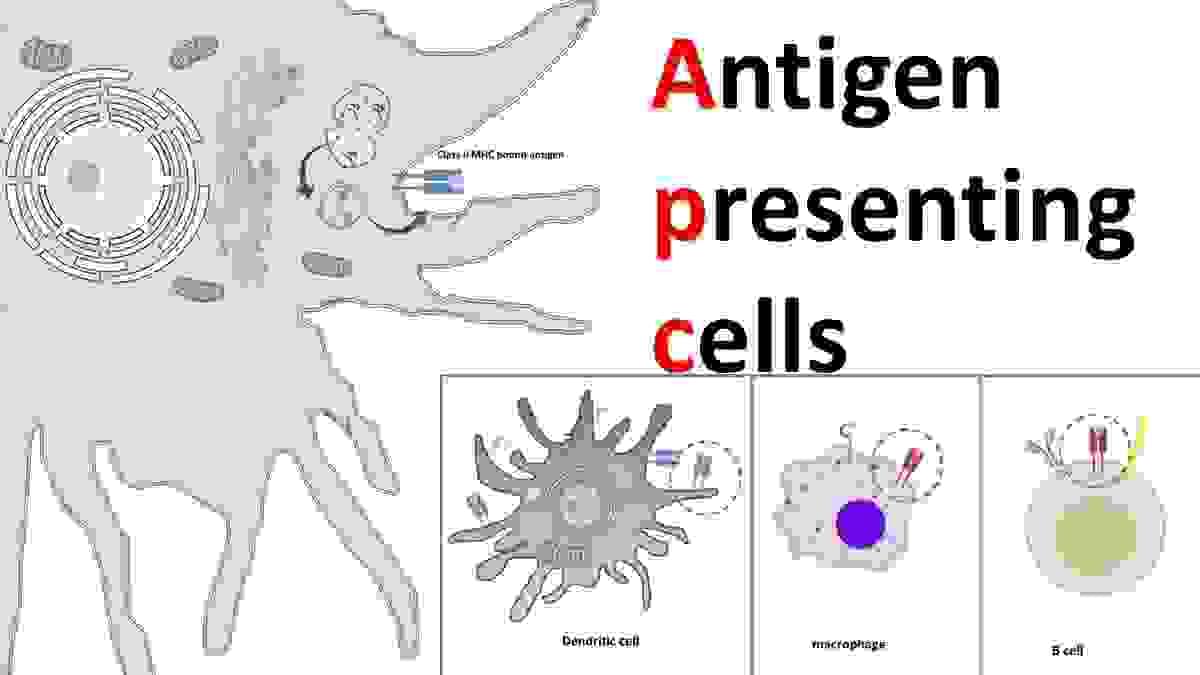
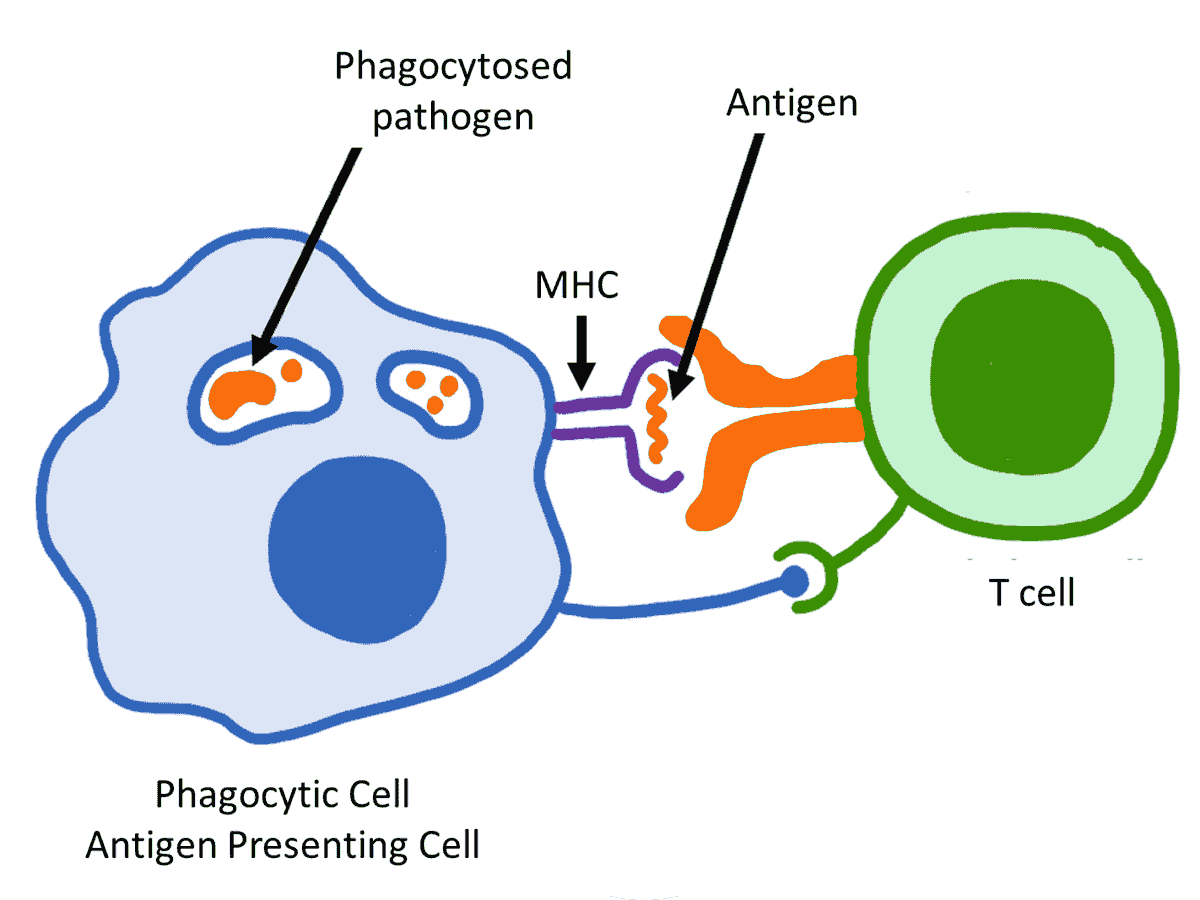
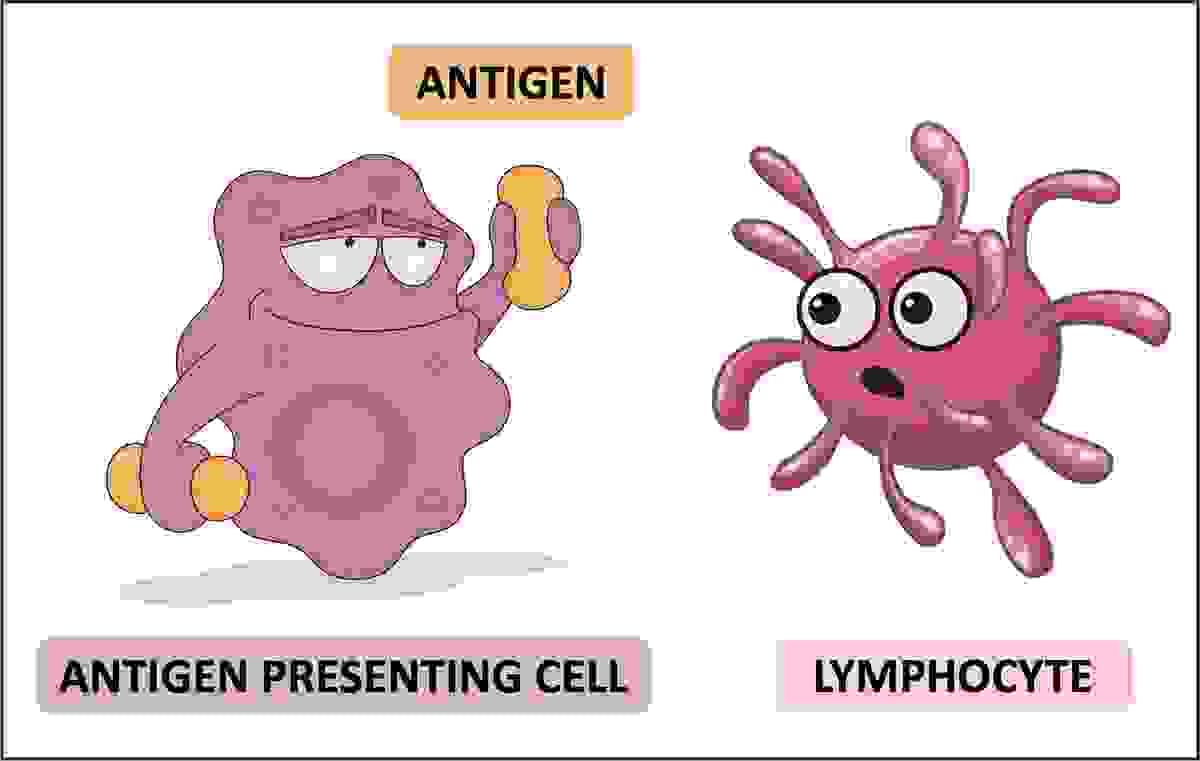
Antigen presenting cell (APC)
An antigen-presenting cell (APC) or accessory cell is a cell that displays an antigen bound by major histocompatibility complex (MHC) proteins on its surface; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T cells.
Almost all cell types can present antigens in some way. They are found in a variety of tissue types. Dedicated antigen-presenting cells, including macrophages, B cells and dendritic cells, present foreign antigens to helper T cells, while virus-infected cells (or cancer cells) can present antigens originating inside the cell to cytotoxic T cells. In addition to the MHC family of proteins, antigen presentation relies on other specialized signaling molecules on the surfaces of both APCs and T cells.
CD4 T cells
Human CD4+ T cells are critical regulators of the immune system, as drastically demonstrated by HIV-infected individuals that develop susceptibility to opportunistic infections and cancer when virus-dependent depletion reduces CD4+ T cell counts below critical thresholds. CD4+ T cells are very heterogeneous in human adults, because they have been generated in response to a high number of different pathogens and belong to a progressively increasing number of different subsets with specialized functions. Helper T cell subsets are defined by the production of cytokines and/or the expression of characteristic lineage-defining transcription factors. Five principal subsets or lineages of CD4+ T cells have been identified so far: T helper (Th)1, Th2, and Th17 cells that target specific classes of pathogens, regulatory T cells that are required to maintain self-tolerance and follicular helper T cells (TFH) that provide help to B cells for antibody production.
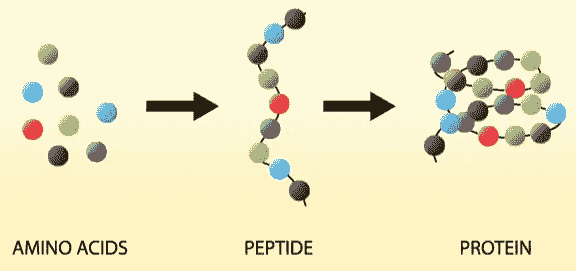
Peptide
Peptides are chains of amino acids (at least two). The acid monomers are connected through peptide linkages and are amongst the most effective known bioactive substances. Examples of such biologically active peptides are hormones, neurotransmitters, or growth factors. The biggest peptide includes up to 100 amino acids. More than 7’000 naturally occurring peptides are known. Longer peptides with three-dimensional shape are called proteins. Peptide synthesis is a chemical process, in contrast to biological substances like antibodies.
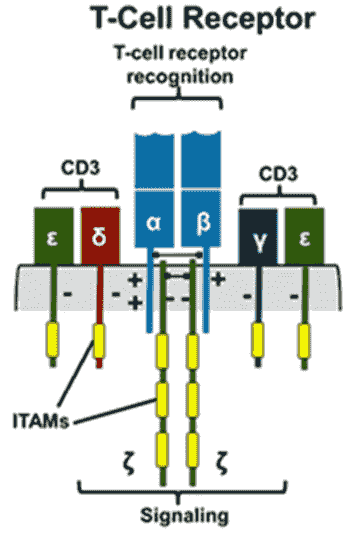
T cell receptor (TCR)
T cell receptors (TCRs) are specific receptors on the surface of T cells that can recognize and bind protein antigens, and are characteristic markers of all T cells. TCR specifically recognizes and binds to specific antigen peptides presented by the major histocompatibility complex (MHC) on the surface of antigen presenting cells (APC) to form TCR-antigen peptide-MHC complex (TCR-pMHC complex) , initiate the first transduction signal, thereby inducing the activation of T cells and exerting adaptive immune effector function.
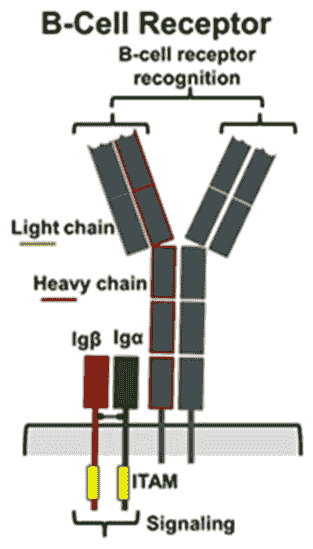
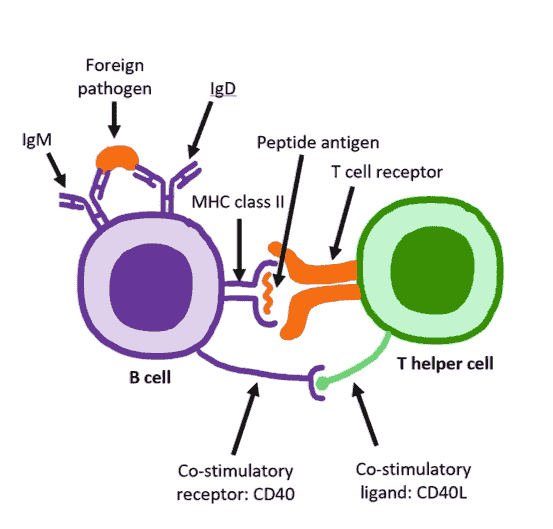
B cell receptor (BCR)
B cell receptor (BCR), also known as B cell antigen receptor, is usually a heterodimeric complex composed of an antigen-binding subunit (membrane surface immunoglobulin, mIg) and a signaling unit. The antigen-binding subunit is a tetramer composed of two heavy chains (H) and two light chains (L) (κ or λ chains), of which the H chain consists of four parts of gene fragments, including 65-100 types of variable regions (VH), 2 types of variable regions (DH), 6 types of binding regions (JH) and constant region (CH); L chain is composed of Three parts of gene fragments, involving variable region (VH), binding region (JH) and constant region (CH) composition. The signaling unit is a heterodimeric protein in which Ig-alpha (Igα, CD79A) and Ig-beta (Igβ, CD79B) are linked by disulfide bonds. The cytoplasmic domain of Ig-alpha is longer and contains 61 amino acids; the cytoplasmic domain of Ig-beta contains 48 amino acids.
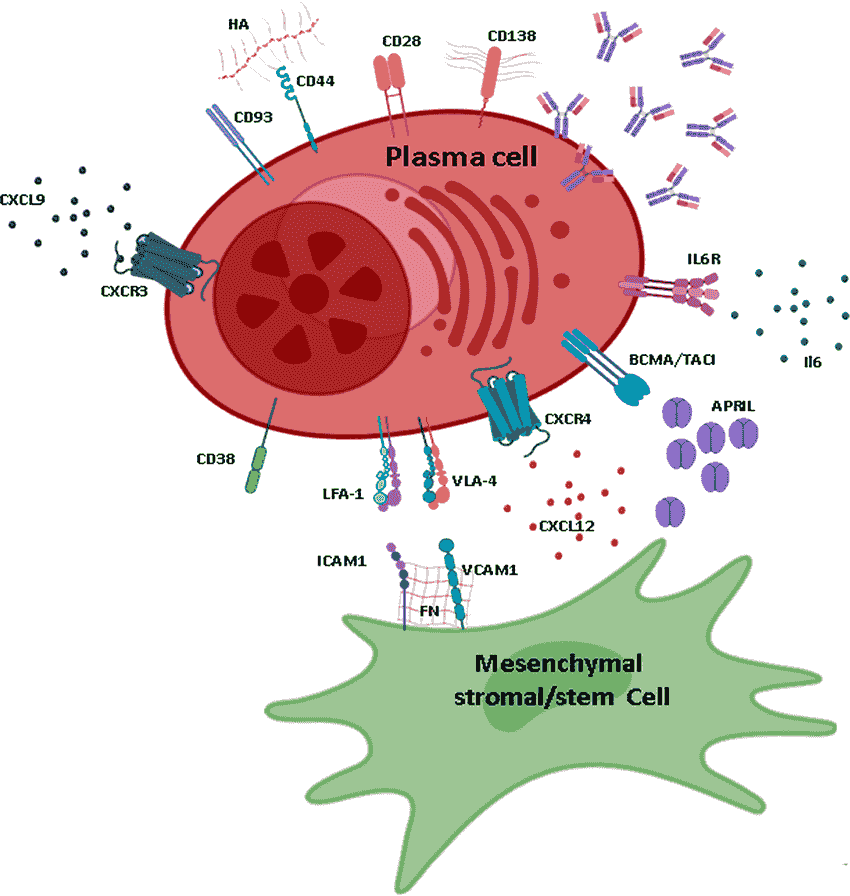
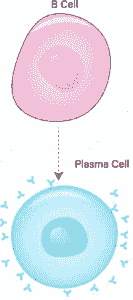
Plasma cell
Also known as plasma B cells, plasma cells are terminally differentiated B lymphocytes. While they originate from activated B cells in the spleen and lymph nodes (secondary lymphoid organs) etc., some plasma cells migrate to the bone marrow where they may persist for an extended period of time.
Here, it's suggested that they interact with the stromal cells that surround the sinusoidal endothelial cells which facilitates the production and release of antibodies into the blood stream.
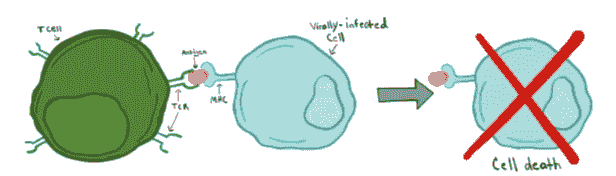
T cell thymic development
T-cells originate from stem cells in the bone marrow and develop in the thymus, a small lymphoid organ located between the lungs. Once in the thymus, immature T cells progress through multiple developmental stages on their road to differentiation into mature T cells capable of recognizing antigens and protecting our bodies from infection. During this period of development, T cells undergo somatic recombination to generate individual T cell clones expressing unique TCRs. These TCRs are key molecules in the identity of each T cell, as they each have the ability to bind and recognize different antigens. In general, this antigen recognition process occurs when the TCR binds to antigen being presented by other cells on MHC proteins (MHC class I in the case of CD8+ T cells, MHC class II in the case of CD4+ T cells). For example, if one of your cells were to be infected by a virus, this infected cell could present viral antigens on its surface via MHC class I molecules, and this antigen-MHC complex would act as a danger signal to the surrounding immune cells. A T cell with a compatible TCR could then bind to the antigen-MHC complex on the infected cell and kill it, thereby preventing the spread of the virus. Given the important role of the TCR in facilitating antigen recognition and cellular killing, it is vitally important that the TCRs produced by somatic recombination 1) are capable of binding MHC complexes and 2) will not recognize our own cells, which also express MHC proteins bound to normal, self-peptides. T cells, then, must walk a very fine line between recognition of that which is foreign and harmful, and that which is self and safe.
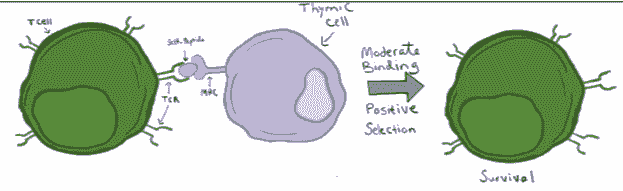
Positive Selection
To address the necessity that T cells be capable of binding MHC complexes, T cells undergo positive selection. In this process, cells in the thymus present short pieces of proteins, called peptides, on their own MHC class I and class II molecules, allowing immature T cells to bind. If TCRs are incapable of binding, the T cell will undergo a type of cell death celled apoptosis. If, however, a T cell’s TCR successfully binds to the MHC complexes on the thymic cells, the T cell receives survival signals and is thus positively selected. Further, this positive selection process also determines if a T cell will become a CD8+ T cell or a CD4+ T cell. Specifically, if a TCR complex binds strongly to MHC class II, the complex will send intracellular signals to induce the expression of a protein called ThPOK. This protein reduces the expression of another key protein, called Runx3, responsible for driving CD8 expression. Because low Runx3 causes low CD8, these ThPOK+, Runx3- cells become CD4+. If, however, a developing T cell does not bind strongly to MHC class II, ThPOK levels will be low and thus Runx3 levels will be high, pushing the T cell to differentiate into a CD8+ cell. In sum, the process of positive selection leads to the survival of mature CD8+ and CD4+ T cells capable of recognizing MHC complexes.
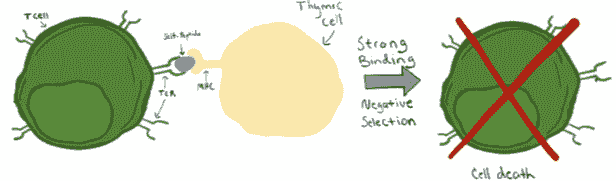
Negative Selection
While the ability of T cells to recognizes antigen-MHC complex is vital for their ability to fight pathogens and other foreign cells, it is equally important that these T cells do not recognize and attack our own cells. This is where negative selection comes into play. As described above, developing T cells in the thymus are presented with peptides bound to MHC molecules, to which they may be able to bind. Importantly, while a moderate degree of binding leads to survival and positive selection, TCRs that bind too strongly to these MHC complexes are destined for the opposite fate (Figure 1, bottom). It is thought that, when TCRs bind too strongly to the MHC complexes in the thymus, the intracellular signaling is so strong that it actually leads to cell death, thereby eradicating immature T cell that have a high likelihood of being self-reactive and attacking our own cells.
One of the most intriguing aspects of negative selection is that it primarily occurs in the thymus, which means that T cells rely solely on the cells in the thymus to present self-peptides on MHC molecules. Because of this, it is tempting to think that negative selection will only delete T cells who show reactivity to thymic self-peptides… but what about peptide-MHC complexes specific to the stomach or the skin or the lungs? Would the T cells that survive negative selection leave the thymus only to kill cells of our other organs? Clearly, this is not the case, and the reason is attributed to a protein called autoimmune regulator, or AIRE. The role of AIRE in the thymus is to induce the expression of many proteins that are not typically expressed in thymic cells, such as proteins characteristic of the lungs. In this way, developing T cells are exposed to many peptide-MHC complexes, not just those normally expressed by thymic cells, thereby preventing autoimmunity once T cells leave the thymus.
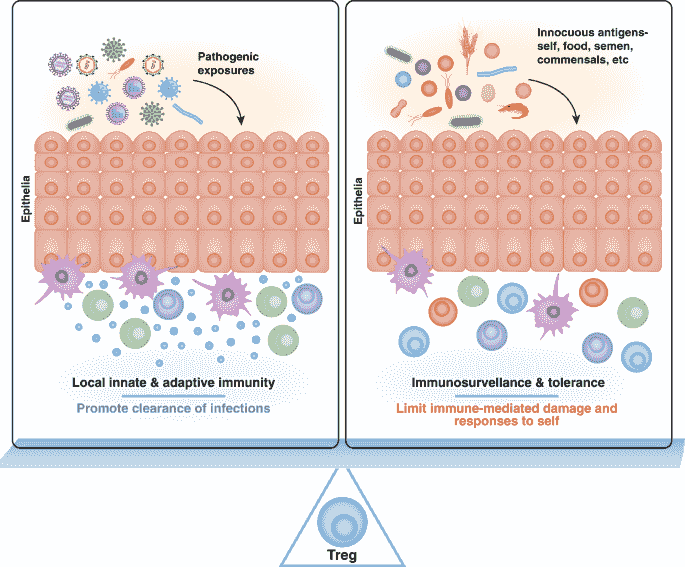
Immune tolerance
Immune tolerance, also known as immunological tolerance or immunotolerance, refers to the immune system's state of unresponsiveness to substances or tissues that would otherwise trigger an immune response. It arises from prior exposure to a specific antigen and contrasts the immune system's conventional role in eliminating foreign antigens. Depending on the site of induction, tolerance is categorized as either central tolerance, occurring in the thymus and bone marrow, or peripheral tolerance, taking place in other tissues and lymph nodes. Although the mechanisms establishing central and peripheral tolerance differ, their outcomes are analogous, ensuring immune system modulation.
Immune tolerance is important for normal physiology and homeostasis. Central tolerance is crucial for enabling the immune system to differentiate between self and non-self antigens, thereby preventing autoimmunity. Peripheral tolerance plays a significant role in preventing excessive immune reactions to environmental agents, including allergens and gut microbiota. Deficiencies in either central or peripheral tolerance mechanisms can lead to autoimmune diseases, with conditions such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes, autoimmune polyendocrine syndrome type 1 (APS-1), and immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) as examples. Furthermore, disruptions in immune tolerance are implicated in the development of asthma, atopy, and inflammatory bowel disease.
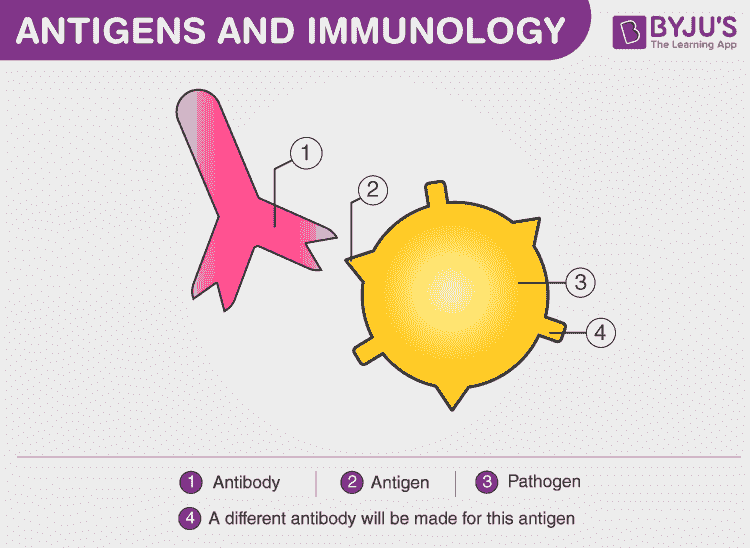
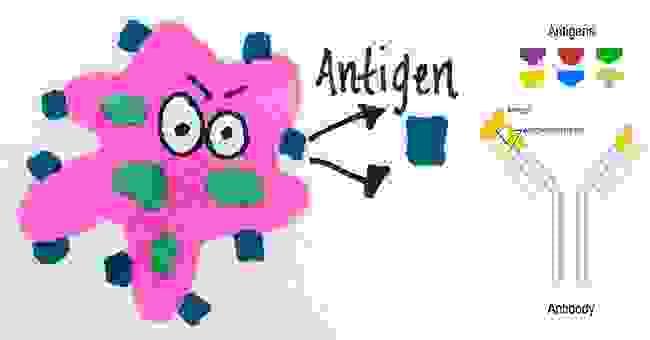
Antigen
Antigens are large molecules of proteins, present on the surface of the pathogen- such as bacteria, fungi viruses, and other foreign particles. When these harmful agents enter the body, it induces an immune response in the body for the production of antibodies.
The immune system has the capacity to distinguish between body cells (‘self’) and foreign materials (‘non-self’)
It will react to the presence of foreign materials with an immune response that eliminates the intruding material from the body
All nucleated cells of the body possess unique and distinctive surface molecules that identify it as self.
These self markers are called major histocompatibility complex molecules (MHC class I) and function as identification tags.
The immune system will not normally react to cells bearing these genetically determined markers (self-tolerance)
Any substance that is recognised as foreign and is capable of triggering an immune response is called an antigen (non self)
Antigens are recognised by lymphocytes which bind to and detect the characteristic shape of an exposed portion (epitope)
Lymphocytes trigger antibody production (adaptive immunity) which specifically bind to epitopes via complementary paratopes
Antigenic determinants include:
- Surface markers present on foreign bodies in the blood and tissue – inluding bacterial, fungal, viral and parasitic markers
- The self markers of cells from a different organism (this is why transplantation often results in graft rejection)
- Even proteins from food may be rejected unless they are first broken down into component parts by the digestive system
Properties of Antigens
- The antigen should be a foreign substance to induce an immune response.
- The antigens have a molecular mass of 14,000 to 6,00,000 Da.
- They are mainly proteins and polysaccharides.
- The more chemically complex they are, the more immunogenic they will be.
- Antigens are species-specific.
- The age influences the immunogenicity. Very young and very old people exhibit very low immunogenicity.
Types of Antigens
Exogenous Antigens
Exogenous antigens are the external antigens that enter the body from outside, e.g. inhalation, injection, etc. These include food allergen, pollen, aerosols, etc. and are the most common type of antigens.
Endogenous Antigens
Endogenous antigens are generated inside the body due to viral or bacterial infections or cellular metabolism.
Autoantigens
Autoantigens are the ‘self’ proteins or nucleic acids that due to some genetic or environmental alterations get attacked by their own immune system causing autoimmune diseases.
Tumour Antigens
It is an antigenic substance present on the surface of tumour cells that induces an immune response in the host, e.g. MHC-I and MHC-II. Many tumours develop a mechanism to evade the immune system of the body.
Native Antigens
An antigen that is not yet processed by an antigen-presenting cell is known as native antigens.

WP1302
Wp1302 can directly bind to MHC class IIextracellularly, inhibiting TSHR-specific autoimmune cells and blocking the
production of TSHR autoantibodies, thereby offering a cue opportunity for the
Graves' disease treatment.WP1302 (ATX-GD-59) consists of twopeptides, S-001 and S-002, derived from key epitopes within the human TSHR,
targeting the major histocompatibility complex (MHC) class II binding site.
WP1302 has high solubility and does not induce cellular endocytosis or
inflammation. WP1302 mediates the interaction between inactive/immature immune
cells (those lacking co-stimulatory molecules) via MHC II, inducing TSHR
specific immune tolerance. WP1302 will suppress the TSHR-specific T cells and B
cells, reduce the production of stimulating autoantibodies against TSHR.WP1302
WP1302 comprises two soluble syntheticlinear peptides, S-001 and S-002, and is presented as a lyophilized powder for
reconstitution as a solution for subcutaneous injection.The TSHR ECD is of primary importance forthe high affinity binding for TSH and TSAb, and also the most important region
for identification of potential apitopes. Therefore, we have focused on the
TSHR ECD. HLA-DRB1*0301-transgenic (HLA-DR3tg) and HLA-DRB1*0401-transgenic
(HLA-DR4tg) mice have been identified as the preclinical animal model for
studying mechanism of action and pharmacological effects of WP1302.S-001 and S-002 were identified as thepreferred epitopes using our proprietary technology platform through a series
of in vitro and in vivo studies. First, the antigen TSHR was subdivided into
overlapping 30-mers and screened to determine the immunogenic regions within
the TSHR ECD (aa20-418), by testing their capacity to elicit an immune response
in HLA-DR3tg and HLA-DR4tg mice: After immunization of HLA-DR3tg and HLA-DR4tg
mice with TSHR, the overlapping peptides were used to screen the T cell responses
in an in vitro T cell proliferation assay. Several regions were found to be
immunogenic. To further investigate the T cell specificity of the peptides, a
collection of T cell hybridomas was produced. The longer peptide regions were
shortened using 15 amino acid long peptides, and a short list of core peptide
sequences was selected through the results of T-cell hybridoma analysis. The
short list of peptides identified was further tested to determine their ability
to bind to the HLA-DR molecules in an in vitro assay. In parallel with the
HLA-DR3tg and HLA-DR4tg mice system and in vitro activities, the TSHR ECD was
analyzed to confirm that Graves’ Disease patients’ PBMCs recognize the two peptides.Adequate solubility is one of keycharacteristics of the immunogenicity of a peptide. Therefore, peptide
development candidates need to be soluble in aqueous solutions to be
efficacious in inducing tolerance.The native sequence of S-001 is insolublein aqueous solution and therefore has been modified by replacing the N-terminal
isoleucine with a lysine residue and adding three charged lysine amino acid
moieties in the N and C-terminal end to meet the solubility criteria. During
the process of modification, the Apitope functionality was monitored in a
series of assays to secure biological function. The modified peptide sequence
S-001 has been analyzed for homology with other protein sequences in humans and
other species (using the Basic Local Alignment Search Tool – BLAST) but no significant match beside identity to TSHR wasobserved.Peptide S-002 fulfilled the Sponsor’s solubility criteria without the need for modification, andtherefore is included as the native sequence in T-cell tolerance induction
study by WP1302 in HLA-DR transgenic mice.There are a number of advantages that theplatform offers over other less specific approaches to induce tolerance (e.g.,
anti-CD3 antibodies). These include:1. Thechosen epitopes have favorable properties for long-term treatment, including
low toxicity, rapid clearance, and a high specificity.2. Insteadof using full-length autoantigens that risk hypersensitivity reactions, this
method employs short peptides based on T cell epitopes, reducing the risk of
severe allergic responses.3. Thesepeptide epitopes are soluble, preventing the formation of aggregates that could
trigger inflammatory responses.4. Theplatform's main safety advantage is its specificity: targeting specific
antigens rather than broadly suppressing the immune system. This has been
validated in both pre-clinical studies and clinical trials, including trials
with ATX-MS-1467 and other peptides (Table 1).Table 1 Summary of the clinicalstudies undertaken using therapeutic peptides that act via MHC Class II protein
Condition
Peptide
Reference
Graves’ Hyperthyroidism
ATX-GD-59
Pearce et al., 2019
Multiple Sclerosis
Glatiramer acetate (Copaxone)
Copaxone SmPC, 2015
Multiple Sclerosis
ATX-MS-1467
Streeter et al., 2015
Chataway et al., 2018
Type 1 Diabetes (TI.D.)
Proinsulin derived peptide C19-A3
Ali et al., 2017
Rheumatoid Arthritis
dnaJP1 derived from bacterial Heat shock protein (HSP)
Prakken et al., 2004
Systemic lupus erythematosus
Lupuzor
Zimmer et al., 2013
Coeliac Disease
Three immunodominant epitopes for gluten-specific
CD4-positive T cells (NPL001,
NPL002, and NPL003)
Goel et al., 2017
Bee Venom Allergy
T cell epitope peptides of the phospholipase A2 (PLA)
Müller et al.,1998

WP1302
Abstract
Thyroid eye disease (TED) remains challenging for clinicians to evaluate and manage. Novel therapies have recently emerged, and their specific roles are still being determined. Most patients with TED develop eye manifestations while being treated for hyperthyroidism and under the care of endocrinologists. Endocrinologists, therefore, have a key role in diagnosis, initial management, and selection of patients who require referral to specialist care. Given that the need for guidance to endocrinologists charged with meeting the needs of patients with TED transcends national borders, and to maximize an international exchange of knowledge and practices, the American Thyroid Association and European Thyroid Association joined forces to produce this consensus statement.
Keywords: thyroid eye disease, consensus statement, American Thyroid Association, European Thyroid Association
1. Summary of Key Points 1440
2. Introduction 1441
2.1 Methods 1442
3. Background 1442
3.1. Epidemiology 1442
3.2. Natural history 1443
3.3. Pathogenesis 1443
3.4. Risks for TED development and opportunities for prevention 1443
3.5. Early diagnosis and referral for TED specialty care 1443
3.6. Role of endocrinologists and ophthalmologists in the care of patients with TED 1444
4. Patient Assessment 1445
4.1. Assessing disease activity and severity 1445
4.2. Assessment of quality of life 1446
4.3. Formal ophthalmology evaluation 1446
4.4. Imaging 1448
5. Overall Approach to Therapy 1449
5.1. Local and lifestyle measures 1449
5.2. Overview of systemic medical and surgical treatments for TED 1449
5.3. Setting for TED care 1449
5.4. Referral to ophthalmology 1449
6. Therapy for Mild TED 1451
6.1. Medical therapy for mild TED 1451
6.2. Surgery for minimal changes in proptosis and lid retraction 1451
7. Management of Moderate-to-Severe TED 1451
7.1. Medical therapies 1451
7.1.1. Glucocorticoids 1453
7.1.2. Therapies for patients with moderate-to-severe TED unresponsive or intolerant to intravenous glucocorticoids 1456
7.1.3. Teprotumumab 1457
7.1.4. Rituximab 1458
7.1.5. Mycophenolate 1459
7.1.6. Tocilizumab 1460
7.1.7. Other agents 1461
7.1.7.1. Other agents tested in TED patients and clinically available 1461
7.1.7.2. Other agents under investigation in TED patients but not clinically available 1461
7.1.7.3. Other agents tested in GD patients with potential benefit in TED but not clinically available 1461
7.2. Radiotherapy for moderate-to-severe TED 1461
7.3. Surgical intervention for inactive moderate-to-severe TED 1462
7.3.1. Surgical intervention overview 1462
7.3.2. Orbital decompression 1462
7.3.3. Strabismus procedures 1463
7.3.4. Eyelid procedures 1463
8. Therapy for Sight-Threatening TED 1463
8.1. Intravenous glucocorticoids 1463
8.2. Radiotherapy in dysthyroid optic neuropathy 1464
8.3. Orbital decompression for dysthyroid optic neuropathy 1464
9. Overview of the Management of TED 1465
10. Research Gaps in the Management of TED 1465
1. SUMMARY OF KEY POINTS
1.1. Diagnosis and assessment
Key Point 3.1: Early diagnosis of TED and simple measures to prevent TED development or progression should be pursued.
Key Point 3.2: Endocrinologists managing patients with Graves' disease should identify referral pathways that ensure patient access to TED specialty care.
Key Point 3.3: Ophthalmologists are key to the management of TED and should always be involved in the care of patients with moderate-to-severe and sight-threatening TED.
Key Point 4.1.1: Endocrinologists should be familiar with basic elements of a TED examination enabling assessment of both activity and severity.
Key Point 4.1.2: Assessment of patients with TED should include activity, severity (with particular attention to impaired ocular motility and visual loss), trend across time, and impact on daily living.
Key Point 4.2.1: The physical and psychosocial impact of TED should be assessed for each patient, as it informs treatment decisions. When formal quantification of quality of life (QOL) is deemed appropriate, Graves' orbitopathy-quality of life (GO-QOL) is the preferred instrument.
Key Point 4.4.1: Orbital imaging using contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is preferred for atypical or severe cases of TED to help determine activity and to exclude other etiologies that could be confused with TED.
Key Point 4.4.2: Noncontrast CT is the preferred modality in patients with TED who are being considered for surgery.
1.2. Initial care and referral for specialty care
Key Point 5.1.1: Local ocular measures and lifestyle intervention should be offered to all patients with TED. Lubricants and nocturnal eye masks may be used to prevent or treat corneal exposure. Ocular occlusion and prisms may be offered to relieve diplopia. The importance of smoking reduction or cessation should be explained, and smokers offered support for this goal.
Key Point 5.3.1: Input from both endocrinologists and ophthalmologists with TED expertise is recommended for optimal management in patients with moderate-to-severe and sight-threatening TED.
Key Point 5.4.1: An ophthalmologist should be consulted when the diagnosis of TED is uncertain, in cases of moderate-to-severe TED, and when surgical intervention needs to be considered. Urgent referral is required when sight-threatening TED is suspected or confirmed.
Key Point 6.1.1: A single course of selenium selenite 100 μg twice daily for 6 months may be considered for patients with mild, active TED, particularly in regions of selenium insufficiency.
Key Point 6.2.1: The clinician should regularly assess the psychosocial impact of concerns about appearance.
1.3. Therapy of moderate–severe TED
Key Point 7.1.1: Infusion therapies for TED should be administered in a facility with appropriate monitoring under the supervision of experienced staff. Awareness and surveillance for adverse side effects are recommended throughout the treatment period.
Key Point 7.1.2: Clinicians should balance the demonstrated efficacy of recently introduced therapies against the absence of experience on sustained long-term efficacy, safety, and cost-effectiveness.
Key Point 7.1.1.1: Intravenous glucocorticoid (IVGC) therapy is a preferred treatment for active moderate-to-severe TED when disease activity is the prominent feature in the absence of either significant proptosis (see Section 2.1. for definition) or diplopia.
Key Point 7.1.1.2: Standard dosing with IVGC consists of intravenous methylprednisolone (IVMP) at cumulative doses of 4.5 g over ∼3 months (0.5 g weekly × 6 weeks followed by 0.25 g weekly for an additional 6 weeks).
Key Point 7.1.1.3: Poor response to IVMP at 6 weeks should prompt consideration for treatment withdrawal and evaluation of other therapies. Clinicians should be alert for worsening diplopia or onset of dysthyroid optic neuropathy (DON) that have occurred even while on IVMP therapy.
Key Point 7.1.1.4: A cumulative dose of IVMP >8.0 g should be avoided.
Key Point 7.1.2.1: Rituximab (RTX) and tocilizumab (TCZ) may be considered for TED inactivation in glucocorticoid (GC)-resistant patients with active moderate-to-severe TED. Teprotumumab (TEP) has not been evaluated in this setting.
Key Point 7.1.3.1 TEP is a preferred therapy, if available, in patients with active moderate-to-severe TED with significant proptosis (see Section 2.1. for definition) and/or diplopia.
Key Point 7.1.4.1: Evidence from randomized controlled trials (RCTs) is limited and divergent but suggests efficacy of RTX for inactivation of TED and prevention of relapses at >1 year, particularly in patients with TED of <9 months' duration.
Key Point 7.1.4.2: RTX therapy is acceptable in patients with active moderate-to-severe TED and prominent soft tissue involvement.
Key Point 7.1.6.1: TCZ is an acceptable treatment for TED inactivation in GC-resistant patients with active moderate-to-severe disease.
Key Point 7.2.1: Radiotherapy (RT) is a preferred treatment in patients with active moderate-to-severe TED whose principal feature is progressive diplopia.
Key Point 7.2.2: RT should be used cautiously in diabetic patients to avoid possible retinopathy. It is relatively contraindicated for those younger than 35 years of age to avoid a theoretical lifetime risk of tumors developing in the radiation field.
Key Point 7.3.1.1: Surgery for moderate-to-severe TED should be performed by an orbital surgeon experienced with these procedures and their complications.
Key Point 7.3.1.2: Rehabilitative surgery for moderate-to-severe TED should only be performed when the disease is inactive and euthyroidism has been achieved and maintained.
Key Point 7.3.2.1: The specific surgical approach should be tailored to the indication (DON, proptosis), type of orbitopathy (muscle or fat predominant congestive disease), and desired reduction in proptosis.
Key Point 7.3.3.2: In patients with diplopia and inactive TED, binocular single vision in the primary position of gaze may be restored with strabismus surgery or permanent prisms ground into the spectacle lenses.
Key Point 7.3.4.1: Eyelid retraction and fat prolapse are surgically corrected when TED is inactive and euthyroidism is achieved, and after surgical decompression and strabismus surgery as indicated.
1.4. Therapy of sight-threatening TED
Key Point 8.1.1: Patients with DON require urgent treatment with IVGC therapy, with close monitoring of response and early (after two weeks) consideration for decompression surgery if baseline visual function is not restored and maintained with medical therapy.
Key Point 8.2.1: RT may be considered for preventing or as an adjunct to treating DON.
Key Point 8.3.1: In patients with compressive DON, orbital decompression of the deep medial wall and orbital floor should be considered to restore vision by reducing apical compression on the optic nerve.
2. INTRODUCTION
Thyroid eye disease (TED) is an autoimmune condition closely related to Graves' disease. It is characterized by endomysial interstitial edema, expansion, and proliferation of cells within the fibrofatty compartment, resulting in the clinical manifestations of periorbital edema, lid retraction, proptosis, diplopia, corneal breakdown, and in rare cases optic nerve compression. TED remains challenging for clinicians to evaluate and manage. Novel therapies have recently emerged, and their specific roles are still being determined.
Most patients with TED develop eye disease while being treated for hyperthyroidism under the care of endocrinologists. Endocrinologists, therefore, have a key role in diagnosis, initial management, and selection of patients who require referral to specialist care. Given that the need for guidance to endocrinologists charged with meeting the needs of patients with TED transcends national borders, and to maximize an international exchange of knowledge and practices, the American Thyroid Association (ATA) and European Thyroid Association (ETA) joined forces to produce this consensus statement (CS).
The scope was to address clinical assessment, to develop criteria for referral to specialty care and treatment, and to focus on medical and surgical treatment in nonpregnant adults (age ≥18 years) with TED. This CS is primarily aimed at endocrinologists and, in particular, those involved in the management of nonpregnant adult (>18 years) patients with TED. A CS was selected as the forum, rather than a clinical practice guideline, to provide a concise and timely appraisal of a rapidly changing therapeutic arena.
In line with the official policies of the ATA and ETA, this CS is intended as an aid to practicing endocrinologists. It does not establish a standard of care, replace sound clinical judgment, or capture all nuances likely to be present in any particular patient; specific outcomes are not guaranteed. We recommend that treatment decisions be based on independent judgments of health care providers carefully considering each patient's individual circumstances such as comorbidities, functional status, goals of care (established at the outset and revisited frequently), and feasibility considerations, including regional access to specific health care resources. Our recommendations are not intended to supplant patient directives.
A recent survey of ATA and ETA members1 found that 53% reported no access to a multidisciplinary clinic, and the cost of some medical treatments was deemed to be a barrier. The CS has taken this important information into account and has striven to achieve a balance between the limitations imposed by the above constraints and encouraging best practice.
2.1. Methods
Membership in the task force (TF) included physicians with expertise in thyroidology and TED, and adherence to the rules of the ATA and ETA on conflicts of interest (https://www.thyroid.org/wp-content/uploads/members/fin-disclosure-coi-policies-2018.pdf; https://www.eurothyroid.com/files/download/ETA-Rules-for-Guidelines-2016.pdf). Cochairs were nominated by ATA and ETA leadership and invited to suggest up to four additional individuals to represent the ATA and ETA. Potential members were discussed and vetted with ATA and ETA society leadership before the final taskforce was assembled.
A series of twice-monthly virtual meetings of the TF with an average attendance of 88% of members took place between January and November 2021, complemented by additional communications. A literature search of PubMed was initially conducted of English language publications from January 1990 through January 2021 and continuously updated up until the time of publication, using the search terms “thyroid eye disease” or “Graves' orbitopathy” or “Graves' ophthalmopathy” or “thyroid-associated eye disease.” References were imported into EndNote and the final database included 3952 unique references. The scope was discussed, agreed upon, and endorsed by the ATA and ETA. A detailed list of subtopics was constructed with approximate word and reference limits assigned to writing groups based on expertise.
Section drafts were reviewed by the TF. Recommendations were listed as “Key Points,” and discussed and modified until full consensus was reached. Specifically, for topics in which there were differing views among taskforce members, a comprehensive discussion took place, allowing iterative modification of the topic content until there was unanimous consensus. The final drafts were approved by the entire TF. Two patient-led organizations, the Graves' Disease and Thyroid Foundation and the Thyroid Organization of the Netherlands, were invited to review the final draft.
In addition, the CS was posted on the ATA and ETA websites for comments and feedback from members. Feedback was also received from the American Academy of Ophthalmology and the American Society of Ophthalmic Plastic and Reconstructive Surgery; the European Society of Ophthalmic Plastic and Reconstructive Surgery was invited to review the CS, but no feedback was received.
The TF chose the descriptor “TED” because it is commonly used in the literature and is meaningful to specialists, generalists, patients, and the general public, although the TF acknowledges that Graves' orbitopathy is also a widely accepted and frequently used term. Multidisciplinary specialized TED care, described hereunder (see Section 3.5), will be referred to as “TED specialty care.”
Several medical therapies are available for TED. Many have not been compared with placebo or compared with one another in randomized controlled studies. Therefore, the TF has categorized treatments as (1) preferable, (2) acceptable, or (3) may be considered, based on its collective interpretation of the available evidence. A treatment is listed as “preferred” if more than or equal to two RCTs have shown efficacy against standard of care or placebo with concordant results; “acceptable” when there exist more than or equal to two RCTs with discordant results but the discordance is deemed likely the result of differing inclusion criteria, or only a single RCT is available and shows efficacy.
Notably, most included RCTs were not placebo-controlled, but, rather, compared with other existing therapies. A therapy is listed as “may be considered” in the case of therapies for which benefit is not clear. Evidence for efficacy in this category may be the result of more than or equal to two RCTs with discordant results that are not easily explicable, or from single RCTs with small efficacy effects, and from larger well-performed observational studies. In general, therapies in the “may be considered” category are utilized in clinical practice only when both preferable and acceptable therapies are unavailable, contraindicated, or the patient is intolerant and/or refuses.
These definitions leave open the possibility of more than one preferable therapy for a given patient, in which case drug availability, cost, and patient acceptability are paramount in selecting the appropriate therapy for a particular patient. The TF is aware that regional differences currently exist in the availability of individual medical therapies and, therefore, some treatments listed as preferable will not be available in all regions of the world.
For therapies selected to reduce proptosis, the TF elected to use the term “significant proptosis” rather than a numerical threshold (i.e., ≥3 mm above the upper limit for race and sex) as a numerical definition would exclude some patients who might otherwise benefit from therapy. In keeping with the definition of moderate-to-severe TED (Table 1), a degree of proptosis <3 mm above the upper limit for race and sex would be regarded as “significant proptosis” if it impacted sufficiently on daily life and would justify the risks of treatment.
Table 1.
Activity and Severity Definitions for Patients with Thyroid Eye Disease
A. Activity 1. Clinical activity score The 7-item CAS is shown hereunder. Each item scores 1 point if presenta Spontaneous retrobulbar pain Pain on attempted up or lateral gaze Redness of the eyelids Redness of the conjunctiva Swelling of the eyelids Inflammation of the caruncle and/or plica (Fig. 2b) Conjunctival edema, also known as chemosis (Fig. 2c) 2. Active TED A CAS ≥3/7 usually implies active TED. A history or documentation of progression of TED based on subjective or objective worsening of vision, soft tissue inflammation, motility, or proptosis is suggestive of active TED independently of the CASB. Severity 1. Sight-threatening TED Patients with DON and/or corneal breakdown and/or globe subluxation (Fig. 2f) 2. Moderate-to-severe TED Patients without sight-threatening disease whose eye disease has sufficient impact on daily life to justify the risks of medical or surgical intervention. Patients with moderate-to-severe TED usually have any one or more of the following: lid retraction ≥2 mm, moderate or severe soft tissue involvement, proptosis ≥3 mm above normal for race and sex, or diplopia (Gorman score 2–3). 3. Mild TED Patients whose features of TED have only a minor impact on daily life insufficient to justify immunosuppressive or surgical treatment. They usually have only one or more of the following: minor lid retraction (<2 mm), mild soft tissue involvement, proptosis <3 mm above normal for race and sex, transient or no diplopia, and corneal exposure responsive to lubricants.
A 10-item CAS is also sometimes used and includes additional points for increase of at least 2 mm in proptosis, decrease of at least 8° in any duction, and decrease of visual acuity by two lines. A limitation of the 10-item CAS is that it requires an earlier assessment of the mentioned measures, which is usually unavailable on first consultation. See Bartalena et al.19
CAS, clinical activity score; DON, dysthyroid optic neuropathy; TED, thyroid eye disease.
3. BACKGROUND
3.1. Epidemiology
There is a close temporal relationship between the onset of hyperthyroidism due to Graves' disease (GD) and TED for patients in whom both disorders occur; in 80% of such cases, both hyperthyroidism and TED develop within 2 years.2 Rarely, TED occurs in euthyroid patients or in those with a history of chronic autoimmune thyroiditis. Notably, TED is almost always seen in conjunction with circulating thyrotropin (TSH) receptor antibodies (TRAbs).3,4
The overall prevalence of TED among patients with GD is up to 40%.5 Recent studies indicate that the clinical phenotype of GD at onset is becoming milder with respect to the prevalence and severity of hyperthyroidism, goiter, and TED.6 Moderate-to-severe and sight-threatening TED now occur in ∼6% and 0.5% of patients with GD, respectively.7 Moreover, TED is a heterogeneous disorder and some clinical variants of the disease (e.g., euthyroid TED) are considered rare.8
3.2. Natural history
The initial description of three phases of TED by Rundle and Wilson remains the widely accepted representation of its natural history.9 An initial active phase is characterized by inflammatory changes, followed by a brief static phase, and lastly by the inactive phase, which patients usually enter 12–18 months after disease onset. Although improvement in signs and symptoms occurs during the latter period, proptosis and extraocular muscle dysfunction frequently do not normalize without intervention and may persist in up to 50% of patients.9
3.3. Pathogenesis
TED develops from an autoimmune-mediated inflammation targeting connective tissue within and around extraocular muscles (EOMs), intraorbital fat, and less frequently lacrimal glands of some patients with GD.2,10 The close link between TED and TRAb supports the hypothesis that the TSH receptor (TSHR) is the primary autoantigen. The insulin-like growth factor-1 receptor (IGF-1R), with which TSHR forms a functional signaling complex on orbital fibroblasts, seems also to be involved in orbital inflammation, adipogenesis, and tissue remodeling.11
The histopathological changes correlate with the natural history and provide a mechanical basis for understanding the clinical features of TED. Infiltration of orbital tissues by lymphocytes and accumulation of hydrophilic glycosaminoglycans, interstitial edema, and increased adipogenesis are the characteristic findings in the active phase of disease. Increased fibrosis and fat infiltration of affected tissues are observed in the inactive phase.2,10
3.4. Risks for TED development and opportunities for prevention
Nonmodifiable risks for the development and severity of TED include older age, male sex, and genetic factors. The potential role of race in TED remains unclear,7 with anatomic differences in both normal and TED orbits postulated to account for variable presentation by race.12
Modifiable risk factors include cigarette smoking, thyroid dysfunction, and the use of radioactive iodine (RAI). Additional potentially modifiable factors are oxidative stress and elevated serum TRAb levels, the latter affected by choice of therapy for hyperthyroidism.7 Epidemiological studies have recently shown that statin therapy is associated with a decreased risk of developing TED in patients with GD.13–15
The use of steroid prophylaxis in those receiving RAI and normalization of thyroid hormone levels and selenium supplementation in those with mild active disease may alter the natural history of TED7 (Fig. 1). Moreover, based on four independent variables (clinical activity score [CAS], serum TRAb levels, duration of hyperthyroidism, and smoking), a quantitative predictive score for identifying patients with GD least likely to develop TED (negative predictive value of 0.91) has been proposed.16 The low positive predictive value (0.28) of this predictive score limits the utility in predicting future TED.
FIG. 1.
Steps to Reduce Morbidity and Improve Quality of Life in Patients with TED. Measures to reduce morbidity associated with TED and improve patients' QOL. (This figure is used and adapted with permission, courtesy of the British Thyroid Foundation, from the Thyroid Eye Disease Amsterdam Declaration Implementation Group UK (TEAMeD) (https://www.btf-thyroid.org/teamed-page) and Dr. Anna Mitchell. The Thyroid Eye Disease Amsterdam Declaration is further described in references 17, 20). Abs, antibodies; GD, Graves' disease; RAI, radioiodine; TED, thyroid eye disease.
3.5. Early diagnosis and referral for TED specialty care
Adoption of a set of simple measures to promote early diagnosis and prevention of TED is recommended by professional organizations,17–19 following the Amsterdam Declaration.20 It is important that endocrinologists have access to specialized clinical services for patients with TED. Five components are essential for optimal management of patients with TED:
Multidisciplinary decision making based on close communication between experts and patients, utilizing shared decision making.
Coordinated care that encompasses the management of both thyroid and orbital disease.
Skills and expertise for the diagnosis, assessment, and treatment by specialists in TED from endocrinology, ophthalmology, orthoptics (for motility testing and prism fitting) and, as needed, otolaryngology/maxillofacial/plastic surgery, clinical psychology/counseling (with expertise in coping skills related to the impairment of QOL related to TED), nuclear medicine, radiology, and radiation oncology.
Availability of evidence-based treatments.
Safe and timely delivery of treatments.
The format of such a service may be a “Combined Thyroid Eye Clinic,”21 variants of this model in a physical or virtual setting, or a combination of both. The organizational details vary between countries and health care systems and are less important than satisfying the mentioned components. While a combined TED clinic structure can promote quality care in a timely manner,22,23 there is no clear evidence that this model of care is superior to others, and delivery of multidisciplinary care is more important than the structure of the clinic.
3.6. Role of endocrinologists and ophthalmologists in the care of patients with TED
Endocrinologists
manage the thyroid dysfunction,
diagnose TED among their patients with GD,
initiate local and lifestyle measures (Section 5.1),
consider checking selenium level (as indicated), 25-hydroxyvitamin D levels, and lipid levels (optional),
refer to ophthalmologists those patients in whom the diagnosis or severity of TED is unclear, and all cases of moderate-to-severe and sight-threatening TED, and
contribute to TED specialty care management decisions including the delivery of systemic therapies, and monitor for adverse events (AEs) of such therapies.
General ophthalmologists:
Diagnose/confirm TED
Provide emergency management of sight-threatening TED after hours
Refer patients with moderate-to-severe or sight-threatening TED to specialty TED care
TED specialty care (Section 3.5)
Diagnose/confirm TED
Medical and surgical management of moderate-to-severe and sight-threatening TED
Ensure optimal management of thyroid disease
Key Point 3.1: Early diagnosis of TED and simple measures to prevent TED development or progression should be pursued.
Key Point 3.2: Endocrinologists managing patients with GD should identify referral pathways that ensure patient access to TED specialty care.
Key Point 3.3: Ophthalmologists are key to the management of TED and should always be involved in the care of patients with moderate-to-severe and sight-threatening TED.
4. PATIENT ASSESSMENT
4.1. Assessing disease activity and severity
A primary objective in the evaluation of TED is to assess factors that inform management and predict outcomes. There is an important distinction in TED between the two interdependent components of inflammatory activity, manifested by pain, redness, and edema, and disease severity, including proptosis, lid malposition, exposure keratopathy (Fig. 2e), impaired ocular motility, and optic neuropathy. The presence of multiple features of inflammation usually signifies active disease. A history of progressive TED further supports the presence of active disease. Definitions of activity and severity are given in Table 1.
FIG. 2.
Composite of selected clinical features in patients with TED. Patient photographs provided with their consent demonstrate (a) lagophthalmos (inability to close eyelid completely); (b) edema and hyperemia of the caruncle (white arrow) and plica (black arrow) (courtesy of P. Perros); (c) chemosis (conjunctival edema) (courtesy of P. Perros); (d) lateral flare due to upper eyelid retraction (courtesy of P. Perros); (e) exposure keratopathy (courtesy of P. Perros); (f) globe subluxation. This is a rare complication in which the eye is displaced anterior to the retracted eyelids. Trapping of the globe may result in painful keratopathy or vision loss. This patient is seen at time of urgent surgery to decompress the orbits and narrow the lid aperture (courtesy of P. Dolman); (g) superior limbic keratoconjunctivitis in eye associated with marked upper lid retraction. This chronic recurring condition is often associated with thyroid disorders and is characterized by enlarged vessels and subepithelial edema involving the superior bulbar conjunctiva and corneal limbus (courtesy of P. Dolman).
When it is unclear whether the disease is active, repeating the assessments after an interval of 4–6 weeks will usually provide the answer, based on a measurable worsening in disease symptoms and signs. The small proportion of patients with TED who subsequently progress to sight-threatening disease can often be identified from the history and examination.24,25 These “high-risk” TED patients are characterized by the features given in Table 2. Such cases merit close follow-up.
Table 2.
Characteristics of High-Risk Thyroid Eye Disease Patients
Background Male sex Age >50 years Tobacco smokerHistory Unstable thyroid function Diabetes mellitus Radioiodine in the past 6 months Progressive symptoms and/or signs of TED Orbital aching DiplopiaExamination Marked soft tissue inflammatory features Lagophthalmos (Fig. 2a) Impaired ocular motility, particularly elevation
The features outlined are associated with an increased probability of developing sight-threatening TED.24
Endocrinologists should be familiar with basic elements of the eye examination for patients with TED as needed to grade severity and activity, according to the worst affected eye. Diagnostic criteria for TED as well as key elements of the eye examination for nonophthalmologists are reviewed in Supplementary Figure S3. A 5-minute patient assessment tool combining subjective and basic objective patient evaluation to diagnose TED and determine a need for ophthalmology referral was found to be efficacious in a pilot trial.26
The most widely used assessment of TED activity is the CAS, adopted by the EUGOGO19 and the ATA clinical practice guidelines on the management of hyperthyroidism.18 A 7-point CAS is currently favored for clinical evaluation that includes pain, erythema, and edema, whereas the 10-point version assesses change over time, using three additional points for worsening proptosis, motility, or visual acuity27 (Table 1). Advantages of CAS include its use of purely clinical parameters and moderate ability to predict response to immunomodulatory therapy.27,28
Examples of CAS elements with patient photographs are provided in open access at (https://onlinelibrary.wiley.com/doi/epdf/10.1046/j.1365-2265.2001.01349.x). Disadvantages include the binary (yes/no) classification in each category, assignment of equal weight to parameters with divergent clinical importance, and being prone to both false positive (congestive orbitopathy) and false negative predictions (aging and darker skin complexion) of response to treatment.29,30
Assessment of TED severity allows an appraisal of the patient's immediate or future threat to vision, a semiquantitative method for determining change over time, as well as for use in research to facilitate interstudy comparison and meta-analysis. Specific ophthalmic measures including visual acuity, ocular motility and alignment, proptosis, and lid retraction can be accurately documented along with their changes in the clinical assessment of TED severity. A widely used method for broadly categorizing TED severity recommended by EUGOGO19 classifies patients as having mild, moderate-to-severe, and sight-threatening disease (Table 1).
Certain clinical parameters indicate a higher risk for development of sight-threatening TED. Features suggesting a threat to vision include spontaneous orbital aching, diplopia, or restriction of eye movements and lagophthalmos (incomplete lid closure), evolving over a period of weeks or months (Fig. 2a).24 In addition, decreased visual acuity, color vision or visual field, a relative afferent pupillary defect (Marcus-Gunn pupil), and optic disk swelling or pallor are indicative of optic neuropathy. Along with the objective changes of the parameters that comprise severity of TED, its impact on daily living should be noted (see Section 4.2, on assessment of QOL).
A comprehensive assessment system for gauging both activity and severity is known as VISA (standing for vision, inflammation, strabismus, and appearance). The VISA Clinical Recording Form (https://thyroideyedisease.org/clinical-visa-recording-forms/) grades both disease severity and activity using subjective and objective inputs. It organizes the clinical measurements of TED into four severity parameters: V (vision, DON); I (inflammation, congestion); S (strabismus, motility restriction); and A (appearance, exposure).
A summary grade for each severity parameter is recorded at the end of the form so that directed therapy may be chosen based on the parameters involved.30 Activity is determined at the first visit by subjective progression in any VISA symptoms over the previous 2 months, or by documented worsening clinical measurements between visits.
Key Point 4.1.1: Endocrinologists should be familiar with basic elements of a TED examination enabling assessment of both activity and severity.
Key Point 4.1.2: Assessment of patients with TED should include activity, severity (with particular attention to impaired ocular motility and visual loss), trend across time, and impact on daily living.
4.2. Assessment of QOL
TED has major negative effects on QOL.31 Impairment in function may negatively impact daily activities (reading, driving, computer work, and watching television), as well as result in dry eye, photophobia, and retro-orbital pain.31 Changes in appearance may lead to psychosocial disability.32–34 In general, the negative effects on QOL correlate with activity and severity and may persist for years.35 The impact of TED on QOL also depends on the specific cultural and psychosocial circumstances of each individual patient and is an important parameter that influences decisions about treatment. Furthermore, the risk-to-benefit ratio of the proposed therapeutic choices should fully encompass the disease impact on the patient's QOL. A widely used and validated QOL instrument is the GO-QOL.31
Key Point 4.2.1: The physical and psychosocial impact of TED should be assessed for each patient, as it informs treatment decisions. When formal quantification of QOL is deemed appropriate, GO-QOL is the preferred instrument.
4.3. Formal ophthalmology evaluation
Ophthalmologists with expertise in TED can confirm the diagnosis and assess severity, activity, and disease trajectory to help plan management. Historical features portending a more severe TED course with diplopia or DON are listed in Table 2.36 A recent onset with rapidly worsening symptoms predicts aggressive disease, requiring expert evaluation, close follow-up, and prompt intervention.37
The directed ophthalmic examination uses standardized techniques to document how the orbit, eye, and eyelids are affected by TED.38 General ophthalmologists can assess vision, ocular motility, and the structures of the eye, and distinguish vision loss from various possible sources, including DON, corneal exposure, astigmatism, or choroidal folds. A subspecialist in oculoplastic and orbital disease will be able to differentiate TED from other orbital conditions, assess imaging, participate in medical management, and perform surgical interventions.
Table 3 organizes the functional and anatomic changes into four clinical categories (vision, soft tissue changes, impairment of ocular motility, and structural changes [proptosis and eyelid malposition]), and lists available ophthalmic techniques and ancillary tests used to assess them.39 For each finding the clinician must consider TED-related causes, non-TED-related causes, or both.
Table 3.
Formal Ophthalmic Examination for Thyroid Eye Disease Based on Vision, Inflammation, Strabismus, Appearance
Clinical ophthalmic examinationAncillary eye testsTED-associated mechanismsNon-TED-associated causesVision
Central vision
Color vision
Peripheral visionSnellen chart
Color plates
Pupil testing
Fundus examinationPattern visual evoked response
Optical coherence tomography (analyzes optic nerve for nerve fiber loss)
Visual field
Corneal topographyDON
Corneal exposure
Dry eye
Choroidal foldsCataract
Macular disease
Glaucoma
Diabetic retinopathyInflammation (soft tissue changes)
Redness and swelling of eyelids and conjunctivaSlit-lamp biomicroscopeClinical photographs
EUGOGOInflammation
Venous congestion
Superior limbic keratoconjunctivitis (Fig. 2g)Allergic infective conjunctivitis
Iritis or scleritis
Dural cavernous fistula
Eyelid margin disease
Eyelid infection or neoplasia
Orbit neoplasia
Orbit inflammationStrabismus (ocular motility changes)
Diplopia
Ductions
StrabismusCorneal light reflex test
(Supplementary Fig. S1a, b)
Cover testingOrthoptics examination:
Perimetric ductions
Field of binocular single vision (area of binocular gaze with single image)
Fresnel prism
Prism measurementsExtraocular muscle restrictionMyasthenia gravis
Dural cavernous fistula
Orbital myositis
Orbital lymphoma
Orbital metastasis
IgG4 disease
Cranial nerve III, IV, VI palsyAppearance (structural changes)
Lid retractionRuler measure
Marginal reflex distance (the distance between the upper lid margin and the corneal reflex when the eye is in the primary position)Clinical photographsUpper lid retraction
Levator scarring
Compensatory levator
Retraction from restricted IR muscle
Lower lid retraction
From proptosis
From IR recession surgeryLid retraction from
Orbital fracture
Maxillary sinus atelectasis ProptosisExophthalmometry Fat expansion
Muscle enlargement
GC-induced lipogenesisOrbital neoplasia
Inflammation
Hemorrhage/trauma
GC-induced proptosis Corneal exposureSlit-lamp biomicroscope
Fluorescein stain Lid retraction
Lacrimal gland inflammationDry eyes
Corneal infection
Eyelid margin diseaseEUGOGO, European group on Graves' orbitopathy; GC, glucocorticoid; IR, inferior rectus.
Visual impairment may be documented by measuring central visual acuity, color perception, and peripheral vision. Dry eyes and corneal exposure impairing vision are identified with the slit-lamp biomicroscope. Features of DON and their prevalence at presentation include color desaturation (98% of DON patients miss two or more plates), central vision loss (90% record 20/40 or less), and relative afferent pupillary defect (Supplementary Fig. S1d) (50%).24 Optic disk edema, hyperemia, or atrophy is rare in DON and their absence does not reduce suspicion or eliminate a diagnosis of DON.24 Perimetry may show visual field defects consistent with optic nerve compression, which might be missed on fundoscopy alone.40
Eyelid and conjunctival edema and redness result from inflammation, corneal exposure, or congestion, and are best assessed with the slit-lamp.41 Rarely, in severe cases, globe subluxation develops, presenting as the equator of the globe protruding beyond the retracted lids (Fig. 2f). Chronic orbital congestion, resulting from impaired venous drainage, may occur independent of active inflammatory changes. Grading is more reliable with clinical photographs or the EUGOGO atlas.42
Restriction of eye movements (ductions) from fibrotic or “tight” EOMs leads to diplopia, typically in upward and lateral gaze. Diplopia is graded from 0 to 3 using the Gorman score (absent, intermittent, inconstant, or constant). Ductions are measured with the light-reflex method (reliable to within 12 prism diopters) (Supplementary Fig. S1a, b).43 Strabismus (ocular deviation) is measured with prisms. An orthoptic evaluation aids in prism fitting and surgical planning.39
Over 90% of TED patients develop upper eyelid retraction. Proptosis is the second most common finding and is measured with the exophthalmometer (Supplementary Fig. S1c); intraobserver reliability with this device is usually within ±1 mm.44 The combination of eyelid retraction and proptosis may lead to corneal exposure, best assessed with the slit-lamp. Upper eyelid retraction is also a feature of thyrotoxicosis of any cause and thyroid status needs to be considered when assessing the position of the upper lids.
Ophthalmological measurements are necessary to fully assess severity and activity of TED. On each follow-up visit, repeat evaluations allow assessment of the disease course (worse, stable, or improving) and response to therapy. This may be facilitated by using a standardized clinical recording form (such as the VISA or EUGOGO forms, downloadable at thyroideyedisease.org or eugogo.eu), which organize the clinical data to permit easy review and comparison between visits.
4.4. Imaging
Orbital imaging is not mandatory for patients with bilateral TED but should be considered in the following situations: (1) to exclude other diagnoses in atypical cases, such as unilateral or euthyroid disease; (2) to assist with assessment in severe cases, in identifying apical crowding, a risk for DON (Fig. 3a, b); (3) to prepare for orbital surgery and in some cases for strabismus surgery (Table 4). Both CT and MRI identify orbital tissue enlargement, including EOMs, orbital fat, and lacrimal glands.45,46
FIG. 3.
Composite clinical–radiographic correlation in patients with TED. Clinical and radiographic image correlations provided with patient consent (courtesy of P. Dolman): (a, b) extraocular muscle enlargement causing periorbital soft tissue congestion, ocular motility restriction, and optic nerve compression with dysthyroid optic neuropathy; (c, d) proptosis in a patient with TED and predominant retroocular fat compartment expansion; (e, f) restricted upward gaze on the right due to right inferior rectus muscle enlargement and fibrosis; (g, h) right upper eyelid retraction and lateral flare due to enlargement and fibrosis of the right levator palpebrae superioris muscle (asterisk).
Table 4.
Primary Indications for Imaging in Suspected or Confirmed Thyroid Eye Disease
Exclusion of other diseases in atypical TED Euthyroid, without history of thyroid dysfunction Clinically unilateral or markedly asymmetric Absent upper lid retraction Upper lid ptosis Atypical strabismus Severe orbital painAssessment in confirmed TED Sight-threatening TED Planning of orbital and in some cases strabismus surgery
Proptosis related to fat compartment expansion alone, without EOM enlargement, can be demonstrated with imaging (Fig. 3c, d). EOM enlargement is typically fusiform with sparing of the tendons and involves, with decreasing frequency, the inferior and medial recti (Fig. 3e, f), superior rectus, or, rarely, all recti and oblique muscles. Levator enlargement as a source of eyelid retraction (Fig. 3g, h) is visible on orbital CT.47
The standard imaging modality is noncontrast CT scan, which is inexpensive, readily available, and allows assessment for decompression surgery. Occasionally, contrast CT is preferred as it shows enhancement of the involved EOM and surrounding fat as an indicator of acute inflammation and may be valuable when a diagnosis other than TED is suspected. MRI provides excellent soft tissue resolution and identifies edema within the muscle on T2 or Short-Tau Inversion Recovery sequence suggesting active disease, but at greater expense, longer imaging duration, and poor definition of the bony walls.45
Other imaging modalities are mainly used in research. When clinical and radiological findings are inconsistent with TED, tissue biopsy of an involved muscle must be considered for exclusion of other pathologies.48 Repeat imaging in patients with TED is generally not required except for the development of new signs or postoperative complications.
Key Point 4.4.1: Orbital imaging using contrast-enhanced CT or MRI is preferred for atypical or severe cases of TED to help determine activity and to exclude other etiologies that could be confused with TED.
Key Point 4.4.2: Noncontrast CT is the preferred modality in patients with TED who are being considered for surgery.
5. OVERALL APPROACH TO THERAPY
5.1. Local and lifestyle measures
In addition to optimally controlling hyperthyroidism as described in clinical practice guidelines,4,18 some nonsystemic treatments and lifestyle measures can be beneficial in TED. Dry eye is common and is caused by corneal exposure and lacrimal gland dysfunction. Corneal exposure occurs due to lid retraction and lagophthalmos (Fig. 2a). Dry eye syndrome (DES) can be treated with artificial tears containing either sodium hyaluronate or carboxymethylcellulose.49 Bland nonmedicated lubricating eye drops, gels, or ointment can be used at night, along with taping of the lids in patients with lagophthalmos, or wearing a headband tightened over a vaseline-moistured eye pad.
Head of the bed elevation, such as sleeping with additional pillows, is sometimes used to relieve edema. Photophobia can be a consequence of DES and is frequently managed with dark glasses and lubricants. Diplopia can be improved with selective ocular occlusion or with Fresnel press-on prisms. Patients should abstain from smoking and avoid second-hand smoke exposure.50
Local and lifestyle measures and watchful monitoring will be sufficient in the majority of patients with mild disease, which in due course will remit completely or partially.51 In selected patients with moderate-to-severe TED, a “watchful monitoring” strategy may also be acceptable. Placebo-controlled studies have shown a 10–59% chance of spontaneous disease inactivation and improvement in proptosis and diplopia in patients who satisfied study criteria for treatment (Table 5).
Table 5.
Efficacy of Pharmacological Therapy for Active Moderate-to-Severe Thyroid Eye Disease
A. Comparisons of outcomes from baseline to after treatmenta,bDrug (ref)Composite outcome (%)Clinical activity score (%)Proptosis (%)Diplopia (%)Disease relapse (weeks)IVGC67,68,71,7223–5345–830–460–1921–40% (week 12)MMF+IVGC686380No changeNo change8% (week 12)–11% (week 24)RTX100831No changeNo change15% (week 40)RTX6760100No changeNo change0% (week 40)TEP917462777029% (week 51)–37% (week 27- see text)TCZ1127393277No dataPlacebo91,100,11210–2222–59No changeNo change0 (week 12)–8% (week 51)
B. Comparisons of treatment outcomes between groupsDrug (ref), n = no. of randomizedComposite outcomeClinical activity scoreProptosisDiplopiaDisease relapse (weeks)MMF vs. GC106
IVGC n = 78, MMF n = 80Favored MMF 79% vs. GC 51%Favored MMF 94% vs. 69%Favored MMF 69% vs. GC 40%Favored MMF 90% vs. GC 64%Favored MMF 0% vs. IVGC 6%MMF+IVGC vs. IVGC68
MMF+IVGC n = 76, IVGC n = 76No difference between groupsNo difference between groupsNo difference between groupsNo difference between groupsNo difference between groupsPost hoc MMF+IVGC 67% vs. IVGC 46%OGC vs. IVGC74
IVGC n = 35, OGC n = 35Favored IVGC 77% vs. OGC 51%Favored IVGC 77% vs. OGC 51%Favored IVGC 60% vs. OGC 40%No difference between groupsFavored IVGC 0% vs. OGC 11% (week 24)RTX vs. IVGC67
RTX n = 15, IVGC n = 16Favored RTX 60% vs. IVGC 38%Favored RTX 100% vs. IVGC 69%No difference between groupsNo difference between groupsFavored RTX 0% vs. IVGC 31% (week 76)RTX vs. placebo100
RTX n = 13, placebo n = 12No difference between groupsNo difference between groupsNo difference between groupsNo difference between groupsNo differences between groups (week 50)Statin+IVGC vs. IVGC71
IVGC, n = 39, IVGC+statin n = 41Favored atorvastatin+IVGC 51% vs. IVGC 28%No difference between groupsNo difference between groupsNo difference between groupsFavored atorvastatin+IVGC 0% vs. IVGC 15%, p = 0.011) (week 24)TEP vs. placebo91
TEP n = 84 placebo n = 87cFavored TEP 74% vs. placebo 14%Favored TEP 62% vs. placebo 22%Favored TEP 77% vs. placebo 15%Favored TEP 70% vs. placebo 31%Data only for TEP 29.4–37% (weeks 27–51)dTCZ vs. placebo112
TCZ n = 15, placebo n = 17Favored TCZ 93% vs. placebo 59%Favored TCZ 73% vs. placebo 29%Favored TCZeNo difference between groupsNo data providedOpen in a new tab
a
Comparisons of efficacy between treatments are subject to bias due to heterogeneity of patient populations, assessment methodology, end points, definitions of response and relapse, and duration of follow-up. The composite outcome is a combination of activity and severity measures and is variably defined. Proptosis improvement was defined as a reduction ≥2 mm in most studies. Diplopia was assessed using the Gorman scoring system.
b
The figures in A represent statistically significant changes compared with baseline, unless marked “no change.”
c
Pooled data from two randomized controlled trials.89,90
d
Data for “flares”/relapses available for TEP group only (not placebo group).
e
Proptosis change from baseline TCZ −1.5 mm versus placebo 0.0 mm.
IVGC, intravenous glucocorticoids; MMF, mycophenolate mofetil; OGC, oral glucocorticoids; RTX, rituximab; TCZ, tocilizumab; TEP, teprotumumab.
Key Point 5.1.1: Local ocular measures and lifestyle intervention should be offered to all patients with TED. Lubricants and nocturnal eye masks may be used to prevent or treat corneal exposure. Ocular occlusion and prisms may be offered to relieve diplopia. The importance of smoking reduction or cessation should be explained, and smokers offered support for this goal.
5.2. Overview of systemic medical and surgical treatments for TED
Decisions concerning treatment beyond local measures are guided by a number of factors including patient symptoms, QOL, disease activity and severity, risk of deterioration, duration of TED, patient age and comorbidity, and patient preference.52 Sight-threatening TED requires urgent treatment, close monitoring of response, and often multimodal treatments.24 In general, treatments during the active phase of TED are aimed at suppressing inflammation and preventing complications and are largely medical. Immunomodulatory treatments are most effective in patients with short duration of TED, the optimal being <6–9 months.53,54
Surgical rehabilitation for proptosis (Supplementary Fig. S2c, d), chronic congestion (Supplementary Fig. S2a, b), strabismus, or lid malposition (Supplementary Fig. S2g, h and i, j) is typically delayed until the quiescent phase,55–57 although urgent surgery may be necessary during the active/progressive phase for DON, severe corneal exposure, or globe subluxation. Systemic medical and surgical treatment for TED are discussed in Sections 6–8.
5.3. Setting for TED care
Optimal management of moderate-to-severe and sight-threatening TED requires a collaborative approach from endocrinologists and ophthalmologists (Section 3.5). Infusion centers, where immunomodulatory therapy may be safely delivered in a controlled setting, vary widely from one institution to the next, but share the common elements of an ability to monitor for and respond rapidly to infusion-related AEs.
Key Point 5.3.1: Input from both endocrinologists and ophthalmologists with TED expertise is recommended for optimal management in patients with moderate-to-severe and sight-threatening TED.
5.4. Referral to ophthalmology
Endocrinologists managing patients with TED should consider referring them for TED specialty care (as defined in Section 2.1 and Section 3.5). Suggested criteria and timing for ophthalmological referral vary according to the clinical presentation of the eye disease, as summarized in Figure 4. The referring endocrinologist will help the ophthalmologist by direct communication, explaining the pertinent clinical features, thyroid status, and risk factors as well as the urgency of referral.
FIG. 4.
Open in a new tab
Referral guidance for patients with TED. Suggested criteria and timing for ophthalmological examination vary according to the clinical presentation of the eye disease (see Section 5.4).
Key Point 5.4.1: An ophthalmologist should be consulted when the diagnosis of TED is uncertain, in cases of moderate-to-severe TED, and when surgical intervention needs to be considered. Urgent referral is required when sight-threatening TED is suspected or confirmed.
6. THERAPY FOR MILD TED
6.1. Medical therapy for mild TED
Selenium has been recommended for patients with mild TED.19 The rationale for the use of selenium centers around its incorporation into selenocysteine-containing proteins, which may have antioxidant and immunomodulatory effects on orbital inflammation.58 In a blinded placebo-controlled multicenter trial conducted in Europe, including geographic areas of marginal dietary selenium intake, patients were randomized to receive 100 μg of selenium selenite twice daily, or placebo for 6 months.59 After 6 months of therapy, improvements in CAS as well as in GO-QOL scores were noted with selenium therapy, but not with placebo, and persisted for an additional six months after therapy was stopped.
Overall, patients treated with selenium were more likely to have improvements in their TED, and less likely to have disease progression.59 Based on the results of this trial, a 6-month course of selenium therapy is recommended for treatment of mild GO of relatively short duration by the EUGOGO,19 and the ETA.4 There is no evidence that selenium provides benefit in patients with moderate-to-severe TED. Selenium selenite contains ∼45% elemental selenium by weight.
Whether selenium therapy is efficacious in selenium sufficient parts of the world remains an important open question. The U.S. recommended daily allowance for selenium is 55 μg daily,60 which is far less than the dose used in mild TED. The potential benefits of selenium supplementation should be balanced against the possible risks of AEs (e.g., possible increased risk of prostate cancer and squamous cell cancers, and type 2 diabetes, though controversial),61 and current evidence does not support extending the duration >6 months.
Key Point 6.1.1: A single course of selenium selenite 100 μg twice daily for 6 months may be considered for patients with mild active TED, particularly in regions of selenium insufficiency.
6.2. Surgery for minimal changes in proptosis and lid retraction
Although mild TED is traditionally defined as having insufficient impact on daily life to warrant immunomodulatory or surgical intervention, even minimal proptosis or lid retraction may project an angry or anxious look, and eyelid fat bulges may give the appearance of premature aging to the face. For some individuals these changes negatively impact their self-confidence and social functioning. Individualized corrective procedures include eyelid narrowing to correct retraction, and blepharoplasties to tighten loose skin and remove fat bulges. The sequence and type of surgery are chosen based on the severity of the changes, the goals of the patient, and the known procedural risks. The indications, timing, and complications of surgery for TED are discussed in Sections 7–8.
Key Point 6.2.1: The clinician should regularly assess the psychosocial impact of concerns about appearance.
7. MANAGEMENT OF MODERATE-TO-SEVERE TED
7.1. Medical therapies
A range of therapies are available for treatment of moderate-to-severe active TED, as supported by evidence from RCTs. Efficacy and safety are key elements in deciding among available therapies. Several therapies require parenteral infusion and premedication to avoid common AEs. Serious AEs can rarely occur during infusion and beyond, making it imperative that these therapies are administered in a safe environment. Individual patient features are important as some treatments are more effective for specific components of TED than others (Table 5).
Appraising the role of different medical therapies is limited by heterogeneity in inclusion criteria (particularly disease activity and duration of TED), and in methods for assessing response to treatment as well as documenting and classifying AEs. The introduction of biologics has raised the cost of treatment many fold over conventional agents. No cost-effectiveness appraisals are available, nor comparative effectiveness trials for any currently available medical therapy for TED. The use of standardized treatment outcomes in clinical trials involving patients with TED has been recently proposed.62 Making decisions about treatment of moderate-to-severe TED lends itself particularly well to the principles of shared decision making. Tables 5–8 and Figure 5 are intended to aid this process.
Adverse Effects of Medical Therapy for Thyroid Eye Disease
Drug (ref)Frequency (%)SeverityaMinor (Grade 1)Moderate (Grade 2)Severe (Grade 3)Life threatening (Grades 4–5)IVGC68,72,74≥10 Hyperglycemia 5–9.9GI symptoms Infection 1–4.9FlushingHypertension depression
Weight gainPsychosis <1 Death, hepatic necrosis, myocardial infarction, strokeOGC66,74≥10GI symptomsHyperglycemia, weight gain, Cushingoid facies Not reported5–9.9 HypertensionInfection 1–4.9 Depression MMF1061–4.9 Infection, hepatitis MMF+GC68≥10GI symptoms Infection 5–9.9 1–4.9 Sleep disorder RTX53,67,100≥10 Infusion reaction (nonsevere) 5–9.9GI symptoms Transient visual lossb 1–4.9 Vasculitis <1 Infusion reaction (severe)TEP89–91≥10GI symptoms, myalgias, alopecia, fatigueHyperglycemiaHearing loss, inflammatory bowel disease aggravation 5–9.9Dry skinTaste disturbance 1–4.9 Cerebral hemorrhageTCZ84,111,112≥10FatigueHyperlipidemia, neutropeniaInfection 5–9.9Pruritus Hepatitis 1–4.9 Thrombocytopenia
Transaminase elevation <1 Anaphylaxis
Bowel perforationcOpen in a new tab
a
National Cancer Institute Common Terminology Criteria for Adverse Events (ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf). Grade 1: Mild, asymptomatic or mild symptoms, clinical or diagnostic observations only, intervention not indicated. Grade 2: Moderate; minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental ADL. Grade 3: Severe or medically significant but not immediately life threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL. Grade 4: Life-threatening consequences, urgent intervention indicated. Grade 5: Death related to adverse event.
b
Believed related to cytokine release syndrome.
c
Observed in other studies (not described in TED studies).
ADL, activities of daily living; GI, gastrointestinal; PML, progressive multifocal leukoencephalopathy.
Table 7.
Logistics of Medical Therapy for Thyroid Eye Disease
DrugRouteFrequency and durationTotal drug cost/full treatment (Euros and U.S. dollars)Ratio of cost of full treatment with drug over cost of full treatment with IVGCaImpact of drug on vaccinationsb€$€$IVGCIV0.5 g weekly for 6 weeks, followed by 0.25 g weekly for 6 weeks€70.0$17211Decreased efficacy of vaccine; live vaccines deferred for 1 month after drug discontinuationOGCPODaily for 3 months (starting with 100 mg prednisolone daily, then tapering dose, cumulative dose 4 g)€73.6$44013Decreased efficacy of vaccine; live vaccines deferred for 1 month after drug discontinuationMMFPO0.72 g daily for 24 weeks€411$1,19167Possible decreased efficacy of vaccine but data are controversialRTXIV1 g two doses 1 weekly for 2 weeks€4,308$19,63662114Decreased efficacy of vaccine; defer vaccination post-therapy until after B cells recovery0.5 g single dose€1,698$4,91424290.1 g single dose€338$99056TEPIVEvery 3 weeks for 6 months (first dose 10 mg/kg, subsequent doses 20 mg/kg, total number of infusions eight)Not licensed in Europe$357,997 for a 75 kg patient51102080UnknownTCZIV8 mg/kg every 4 weeks for 12 weeks (four doses)€4,266$14,5196184Decreased efficacy of vaccine
Open in a new tab
a
Note on relative pricing—A course of IVGC costs €70.0 in Europe and $172 in the United States, derived from (www.pharmacychecker.com). These costs reference the price of medication alone, excluding administration costs. Figures are EU average costs supplied by manufacturers (Roche global) and approved by EMA (personal communication with one of coauthors).
b
Best to complete vaccination series at least 1 month before initiation of all these agents. Data about the impact of various drugs on vaccines are mainly derived from the literature on their use in rheumatological disorders.
IV, intravenous; PO, oral dosing.
Table 8.
Clinical Situations That Favor a Particular Modality as Treatment for Active Moderate-to-Severe Thyroid Eye Disease
Clinical situationIVGC/OGCMMFaRTRTXTEPTCZPatients unresponsive or intolerant to GC = b?√√ /√√c?√Adult patients <35 years of age√√ dX√ d√ d√ dChronic infectioneXX√X√ /√√XLiver disease!/X!√√√ /√√!/XActive gastrointestinal disease!!√!!/X!/XCardiovascular disease!/X√!/X f!/X√√Diabetes mellitusf!/X√!/X√!/X√Chronic kidney disease√!√√√√
√√: favored choice; √: may be favored choice; !: cautious use; = : may be acceptable depending on the clinical circumstances; X: relative contraindication;?: insufficient evidence to recommend for or against treatment. Therapies are presented in alphabetical order.
a
Typically used as combination therapy with IVGC/OGC (please check contraindications to IVGC/OGC therapy).
b
In patients with relapsed TED after OGC or IVGC treatment (cumulative dose 4.5 g), a second cycle of IVGC (cumulative dose <8.0 g) may be considered.
c
May be more efficacious in TED of relatively short duration (<9 months).
d
All Women of childbearing potential must use effective contraception during treatment.
e
Chronic hepatitis, tuberculosis.
f
Diabetic and hypertensive retinopathies are contraindications to RT; uncontrolled diabetes is a contraindication to GC and TEP.
GC, glucocorticoid, RT, radiotherapy.
FIG. 5.
Open in a new tab
Overview of the management of TED. An individualized approach to the management of TED, based on disease activity, severity, duration, trend across time, impact of the disease on daily living, treatment goals, patient age, and comorbidities, as well as the availability and relative costs of therapies, must be advised. Wherever possible, the task force members ranked therapeutic approaches as either “preferred,” “acceptable,” or “may be considered” (see Section 2.1. for definitions). 1See Figure 1. 2Except for the mildest cases improving with local measures. 3See Table 8. 4In most patients with mild TED, a “watchful monitoring” strategy is sufficient (it includes simple measures, see Section 5.1 and Fig. 1). Selected cases (with a significant decrease in QOL) may be treated as moderate-to-severe TED. 5In patients with symptomatic inflammatory soft tissue involvement or if radioactive iodine is used (oral glucocorticoids prophylaxis). 6Particularly in countries that are selenium insufficient. 7Standard treatment—IVGC (cumulative dose 4.5 g). 8In selected patients, a higher cumulative dose of methylprednisolone (7.5 g) may be considered. 9In patients with prominent soft tissue involvement and diplopia. 10In patients with a short duration of TED (< 9 months). 11In patients who are intolerant or resistant to IVGC. 12In selected patients with moderate-to-severe TED, a “watchful monitoring” strategy may be acceptable. 13See Section 7.3.2, and Supplementary Figure S2a, b. 14If there is coexistent active disease, then medical treatment as for moderate-to-severe disease is indicated in parallel with surgical treatment. 15High doses of IVGC (500–1000 mg of methylprednisolone) for 3 consecutive days or on alternate days during the first week. IVGC, intravenous glucocorticoid.
Key Point 7.1.1: Infusion therapies for TED should be administered in a facility with appropriate monitoring under the supervision of experienced staff. Awareness and surveillance for adverse side effects are recommended throughout the treatment period.
Key Point 7.1.2: Clinicians should balance the demonstrated efficacy of recently introduced therapies against the absence of experience on sustained long-term efficacy, safety, and cost-effectiveness.
7.1.1. Glucocorticoids
Mode of action
GCs alter the distribution, survival, and trafficking of leukocytes, interfere with the function of B and T cells, and reduce recruitment of monocytes and macrophages.63
Clinical experience
GCs have been used for >60 years for TED and studied extensively. RCT data have been published on oral glucocorticoid (OGC)64–66 and intravenous GC (IVGC)65–68 from >300 to 500 patients, respectively. Data on IVGC AE in TED are documented for >1200 patients treated.65
Efficacy
There is only one small RCT comparing IVMP with placebo in 16 patients with TED,69 which showed beneficial effects. Data pertaining to the efficacy of IVGC is largely derived from RCTs in which IVGC is compared with other therapies such as OGC,64,70 RTX,67 or to combination therapy including mycophenolate mofetil plus IVGC68 or IVGC plus atorvastatin.71 In addition, a large RCT comparing three different cumulative doses of IVGC provides data on the efficacy of this modality, discussed hereunder.72 Although several nonrandomized studies on GC have been performed,73 this section emphasizes data from relevant RCTs.Activity
Improvement in disease activity, defined variably, occurs in 58–83% of IVGC-treated patients,67,72,74 compared with 51% of those treated with OGC.74 An RCT including 70 patients with active moderate-to-severe TED showed improvement in median CAS values from 5 to 2 with IVGC, versus improvement in CAS values from 5 to 3 in OGC-treated patients.74 Overall, 77% (27/35) of patients treated with IVGC and 51% (18/35) of those treated with OGC experienced improvement in CAS by 3 points. The IVGC arm of another RCT included 81 patients with active moderate-to-severe TED in whom CAS fell from a baseline mean of 3.66 and 3.66 (right and left eyes) to 1.65 and 1.68, respectively, at 36 weeks.68
Another RCT that included an IVGC treatment arm in 16 patients with TED found that 75% had CAS improvement by ≥2 at 24 weeks, and 69% had CAS inactivation to values <3.67 A recent RCT comparing IVGC plus atorvastatin with IVGC alone found that 28% of 39 patients treated with IVGC alone had improvement in a composite outcome.71 Finally, in an RCT involving 159 patients with active moderate-to-severe TED, comparing three IVGC cumulative doses of 2.25, 4.98, and 7.47 g, improvement in CAS >2 points was found at 12 weeks in 81–83% using the two higher dose regimens and 58% of the low-dose treated patients.72 Disease inactivation (defined in this study as CAS ≤2) occurred in 45–65% of patients.Severity
Proptosis is reported to improve by >2 mm at 12 weeks in 20–60% of patients,72,74 but studies with longer term follow-up show no proptosis response.67,68 With regard to diplopia, a range of responses to IVGC have been reported from little overall improvement68 to 57% reduction in constant diplopia at 12 weeks.74 A comparison of IVMP doses showed that a high cumulative dose (7.5 g) was associated with modest improvement in ocular motility (elevation and abduction) in 33% of patients receiving this dose, with no difference in subjective diplopia compared with lower cumulative doses.72 A recent meta-analysis found only small improvements in proptosis and diplopia compared with baseline.75Quality of life
QOL assessments have shown variable improvement from baseline for IVGC.68,72,74 A 2005 study utilizing the SF-36 to assess physical and psychological components of QOL found that an overall rating of good or excellent occurred in 9% of patients at baseline but improved significantly to 80% after therapy.74 A study utilizing the GO-QOL tool has shown improvement of at least 6 points on a 100-point scale in 48–67% of patients after three different cumulative doses of IVGC.72 Results from the IVGC arm of another trial showed improvement of 5–10 points at 24 and 36 weeks compared with baseline.68
Dosing and route of administration
Dosing of IVMP was tested in a large RCT (n = 159) comparing three doses with a finding that a cumulative dose of 4.5 g (administered as 0.5 g weekly × 6 weeks followed by 0.25 g weekly for an additional 6 weeks) was judged to be suitable for most patients with moderate-to-severe TED for disease inactivation.72 Topical GC drops are rarely helpful in TED, and retrobulbar GC injections pose risks of injury to the globe and are less effective than systemic GC.76
Nonresponse and relapses after completion of treatment
Failure to inactivate TED is observed in 20–40% and 40–60% of patients treated with IVGC or OGC, respectively.72,74 Relapse after treatment with different doses of IVMP was studied in a large multicenter study but limited to 12 weeks of follow-up after completion of treatment.72 In this study, relapse, or deterioration, as defined by either the development of DON or at least two additional items among the following: widening palpebral fissure, an increase in soft tissue inflammatory changes by two grades on the NOSPECS system,19 worsening proptosis by ≥2 mm, or increasing restriction in eye movement and/or worsening diplopia, occurred in 31% of patients.
New DON and worsening diplopia can occur despite improvement in inflammation with GC therapy, with DON occurring in 25 of 144 (17%) patients a mean of 5.5 months after starting GC in one retrospective analysis.77 Early TED deterioration78 or unresponsiveness79 after 6–8 weeks of IVGC may predict treatment failure and alternative therapies should be considered.Safety
AEs in relation to IVGC have been reviewed from the published literature relating to a total of 1220 patients.65 A systematic review found that 43 of 101 (42.6%) patients treated with IVGC for TED developed a total of 119 AEs, including 2 events (1.7%) considered major (hepatitis and depression), 49 (41%) moderate, and 68 (57%) classified as minor.73 The risk of death in this study was 0.6%, resulting from cardiovascular and hepatic causes. Common AEs include new or worsened hyperglycemia, worsening hypertension, weight gain, Cushingoid appearance, increased intraocular pressure, insomnia, depression, and psychosis.68,72,74
Major AEs were noted in 6.5% of patients in another large study and are more frequent with higher cumulative doses.72 A cumulative dose of >8.0 g IVMP is associated with a risk of severe hepatotoxicity,73,80,81 and should be avoided. Whether this risk dissipates after a time interval and whether OGCs add to the risk are unknown. The decision to exceed this limit, as in cases of new onset DON, should take careful account of expected benefits balanced against the risks for the individual patient as well as consideration of alternative treatment modalities.
Exclusion of viral hepatitis (by testing for viral DNA) and occult infection, such as tuberculosis, is needed before treatment, particularly for patients with a high risk of such infections. Monitoring for side effects during therapy (Table 6) is required. Contraindications to therapy include active viral hepatitis and hepatic dysfunction, severe cardiovascular disease, uncontrolled hypertension or diabetes, and untreated psychiatric disorders.19Cost
OGC and IVGC are the least costly systemic treatments for TED (Table 7).Summary of evidence
OGC and IVGC have been used and studied extensively in active moderate-to-severe TED.74,82,83 Available evidence shows efficacy for disease inactivation, marginal benefit on eye motility, and negligible benefit on proptosis. AEs are common from GC therapy, but overall, the safety profile is acceptable. The evidence also favors IVGC over OGC.
Key Point 7.1.1.1: IVGC therapy is a preferred treatment for active moderate-to-severe TED when disease activity is the prominent feature in the absence of either significant proptosis (see Section 2.1. for definition) or diplopia.
Key Point 7.1.1.2: Standard dosing with IVGC consists of IVMP at cumulative doses of 4.5 g over ∼3 months (0.5 g weekly × 6 weeks followed by 0.25 g weekly for an additional 6 weeks).
Key Point 7.1.1.3: Poor response to IVMP at 6 weeks should prompt consideration for treatment withdrawal and evaluation of other therapies. Clinicians should be alert for worsening diplopia or onset of DON that have occurred even while on IVMP therapy.
Key Point 7.1.1.4: A cumulative dose of IVMP >8.0 g should be avoided.
7.1.2. Therapies for patients with moderate-to-severe TED unresponsive or intolerant to IVGCs
For patients who do not respond, partially respond, or are intolerant to IVGC therapy, RTX (see Section 7.1.4) and TCZ84 (see Section 7.1.6) may be considered. TEP (see section 7.1.3) has not been evaluated as salvage therapy in this setting. Other options, based on anecdotal evidence, are an additional course of IVGC (in patients with previous partial response, aiming not to exceed 8 g of methylprednisolone), or RT (see section 7.2). For patients whose disease is not progressive and who are not severely symptomatic, watchful monitoring is also an option.
Key Point 7.1.2.1: RTX and TCZ may be considered for TED inactivation in GC-resistant patients with active moderate-to-severe TED. TEP has not been evaluated in this setting.
7.1.3. Teprotumumab
TEP is licensed only in the United States at the time of publication of this CS but is expected to be granted European Medicines Agency license in the future, hence its inclusion in this section.
Mode of action
A role of the IGF-1R in the pathogenesis of TED was suggested in early in vitro studies showing interactions between circulating TSH-R antibodies and the IGF-1R on orbital fibroblasts.85,86 Further evidence regarding the role of TSHR and IGF-1R crosstalk in the pathophysiology of TED emerged over the past decade.87,88
Clinical experience
TEP is the newest agent applied to the management of TED and paradoxically is the only drug approved by the Food and Drug Administration (FDA) for treatment of TED for patients ≥18 years of age, although methylprednisolone has long been FDA approved for “ocular inflammatory conditions unresponsive to topical corticosteroids.” More placebo-controlled trial data are available for TEP than for any other agent in current use, and it appears to be the most comprehensively effective therapy to date (see Efficacy and Table 5). Several important caveats need to be considered (see the Summary of Evidence section)
Efficacy
Early interest in the role of the IGF-1R in TED led to testing TEP, a fully human IGF-1R-inhibitory monoclonal antibody, in two placebo-controlled RCTs in patients with active moderate-to-severe disease.89,90Composite outcome
In the first RCT comparing TEP with placebo, the primary outcome was defined as a composite of improvement in both CAS by ≥2 and reduction in proptosis by ≥2 mm at 24 weeks.89 This outcome was achieved by 69% (29/42) of patients assigned to TEP and 20% (9/45) of those receiving placebo. Among patients with baseline diplopia, there was improvement (defined as a minimum of one grade) in 68% (19/28) versus 29% (8/28) with placebo.Activity
In the first RCT, the mean CAS score improved significantly more in the TEP-treated patients compared with placebo (3.4 vs. 1.85), and 69% of patients receiving TEP experienced disease inactivation to CAS of ≤1, compared with 21% of patients receiving placebo.89 In the second RCT (Treatment of Graves' Orbitopathy to Reduce Proptosis with Teprotumumab Infusions in a Randomized, Placebo-Controlled, Clinical Study [OPTIC]), disease inactivation (CAS ≤1) occurred by 24 weeks in 59% (24/41) of patients versus 21% (9/42) given placebo.90Severity
In the first RCT, proptosis improved from baseline by a mean of 2.5 mm (vs. 0.15 improvement with placebo), and 40% (17/42) experienced proptosis reduction of ≥4 mm, compared with zero patients receiving placebo at 24 weeks.89 In the OPTIC trial, a proptosis reduction of ≥2 mm (the study's primary outcome) was achieved in 83% (34/41) of patients treated with TEP versus 10% (10/42) receiving placebo at 24 weeks, using an intention-to-treat analysis.90 Among patients with baseline diplopia in the OPTIC trial, there was improvement in 68% (19/28) versus 29% (8/28) with placebo.
A pooled analysis combining data on the 84 patients receiving TEP and 87 given placebo in the two RCTs showed a mean improvement in proptosis at 24 weeks of 3 mm in patients receiving TEP versus <0.5 mm in those given placebo. Diplopia improved in 70% (46/66) of patients treated with TEP versus 31% (18/59) of patients given placebo.91 A similar number of patients required additional medical or surgical treatments for TED with TEP (n = 8) and placebo (n = 11).91Quality of life
In the first RCT, the visual functioning QOL improved significantly more with TEP than in the placebo group, whereas the appearance QOL subscale did not.89 In the OPTIC trial, the mean GO-QOL score improved by 13.8 points in TEP-treated patients versus 4.4 points with placebo, with significant improvement in both appearance and visual subscales.90 In the pooled analysis from these two RCTs, the visual function and appearance subscales both improved significantly more with TEP than with placebo (19.7 points vs. 7.0 points and 17.7 points vs. 5.6 points, respectively).91Inactive disease and TED of longer duration
The response to TEP in patients with inactive (CAS ≤1) TED is currently being examined in an RCT (NCT04583735), with results expected in early 2023. A retrospective analysis of 31 patients with a mean TED duration of 81 months, with CAS ≤3 and without changes in diplopia or proptosis for >1 year, who received at least 3 infusions of TEP, found a mean proptosis reduction of 3.5 mm, and 90% (28/31) of patients experienced ≥2 mm reduction.92 Results from Treatment of Graves' Orbitopathy to Reduce Proptosis with Teprotumumab Infusions in an Open-Label Clinical Extension Study (OPTIX-X) provide additional data on the use of TEP in patients with TED of longer duration.93
Among 37 patients treated with placebo in OPTIC, who subsequently received TEP in OPTIC-X, the mean ± SD duration of disease was 12.3 ± 25 months, compared with 6.4 ± 2.4 months duration in OPTIC.93 Proptosis in these patients improved by ≥2 mm in 89% (33/37), diplopia improved in 61% (14 of 23), and CAS improved in 66% (21/32) of those with a baseline OPTIC-X CAS of >1.
Dosing and route of administration
TEP is given intravenously in eight doses, each 3 weeks apart. The first dose is 10 mg/kg, and the seven subsequent doses are 20 mg/kg.
Nonresponse and relapses after completion of treatment
Among the 34 patients showing a proptosis response of ≥2 mm in OPTIC, 10 patients (29.4%) experienced a relapse (described as “flare”) over the ensuing year, including 5 who had a proptosis relapse alone, 4 who experienced both a proptosis and CAS relapse, and 1 with a CAS relapse alone.93 Relapses had occurred at week 48 (27 weeks after final infusion) in seven patients, week 60 in two patients, and week 72 in one patient. The OPTIC-X study also examined the effect of a repeat course of eight TEP infusions in poor responders (n = 5) or those who relapsed after an initial study-defined response (n = 8) in OPTIC.
For the five nonresponders, two responded with proptosis reduction of ≥2 mm, one patient remained a nonresponder, and two dropped out due to either poor response or a serious adverse effect (intracerebral hemorrhage). For 8 patients among the 10 who relapsed after an initial response in OPTIC for whom data from OPTIC-X are available, 5 of 8 experienced proptosis reduction of ≥2 mm with the second course of TEP. An FDA briefing document cites a relapse rate of 37% at 72 weeks among TEP-treated patients (relapse defined as an increase in proptosis of ≥2 mm from week 24 in the study eye only) (https://www.fda.gov/media/133429/download).Safety
TEP should not be used during pregnancy or for patients <18 years of age due to concerns regarding growth. The AE profile of TEP appears to be acceptable, but deterioration of glucose control in patients with diabetes or prediabetes, at times requiring insulin therapy, was noted in 10% of patients.94 Muscle cramps were reported in 25% of patients treated with TEP, nausea in 17%, alopecia in 13%, fatigue in 12%, and, importantly, hearing impairment in 10% of patients.91 A recent summary of five series reported hearing impairment in 29 of 190 (15.2%) patients treated with TEP, with resolution in 16 (55%) but persistence in 13 (45%) patients.95
Aggravation of inflammatory bowel disease (IBD) on TEP was noted in two patients in the two existing RCTs, and apparently new diagnoses of IBD have been described in conjunction with TEP therapy,96 so cautionary use of this drug is recommended in patients with this disorder. In the United States, the drug was granted FDA approval in 2020.Cost
One course consisting of eight infusions of TEP has a retail cost of ∼$300,000, depending on patient weight, ∼2000 times that of IVGC.Summary of evidence
The evidence for efficacy of TEP in patients with active moderate-to-severe TED of short duration with significant proptosis is convincing. However, 17–31% of patients treated did not meet the study definition for a response to treatment, 29–37% experienced disease relapse after an initial response,91,94 and data on improvement in nonresponders after a second course of TEP therapy are quite limited.93 Given lower costs and wider availability, IVGC may be preferred when the treatment target is purely inflammatory changes.
Further data related to TEP therapy, as with other therapies for TED, are needed in the following areas: (1) durability of improvement, (2) efficacy in inactive TED, (3) utility in patients unresponsive to initial therapy, (4) the ability to avoid subsequent medical therapy or rehabilitative surgeries, and (5) long-term safety. Additional trials to determine optimal dosing and duration of treatment, and direct comparisons are needed with other widely available therapies. These unknowns, as well as the high pricing, limited global availability, and absence of cost-effectiveness and comparative effectiveness data, prevent a complete appraisal of TEP's current role in the management of TED.
Cost-effectiveness appraisal is particularly important for TEP, given the high pricing of the drug in comparison with other treatments (Table 7). In the meantime, there is a case for all stakeholders, including professional organizations, insurers, health care providers, patients and their advocates, and drug manufacturers, to engage in discussions on how costly treatments for TED can be made more accessible. The manufacturer of TEP has recently developed a patient-directed cost assistance and insurance process online resource (https://www.tepezza.com/cost-and-support/).
Key Point 7.1.3.1: TEP is a preferred therapy, if available, in patients with active moderate-to-severe TED with significant proptosis (see Section 2.1. for definition) and/or diplopia.
7.1.4. Rituximab
Mode of action
RTX targets CD 20 on activated B cells and impairs new antibody production and B cell-mediated helper function. It has been used extensively for lymphoma and some systemic autoimmune diseases.97
Clinical experience
RTX has been used for TED for the past 15 years. Approximately 160 patients have been reported in the literature to have received RTX for TED.98,99 There are only two small single-center RCTs with a total of 28 patients treated with RTX.67,100
EfficacyActivity
The two RCTs are discordant with regard to the ability of RTX to induce inactivation compared with IVGC or placebo.67,100 The RCT demonstrating efficacy showed CAS decrease from baseline with both treatments (IVGC from 4.7 to 2.2, RTX from 4.4 to 0.6 at 24 weeks), and significantly greater CAS reductions after RTX (n = 15) than after IVGC (n = 16) at 16, 20, and 24 weeks.67 At 24 weeks, disease inactivation (CAS <3) occurred in significantly more RTX-treated patients than in IVMP-treated patients (100% vs. 69%).
The RTX group included patients (40%) who had been previously treated with steroids, but continued to have active moderate-to-severe TED. In the second RCT, RTX (n = 13) was compared with placebo (n = 12) and failed to demonstrate efficacy.100 Observational reports suggest efficacy.98,99Severity
The RCTs and observational studies indicate little to no effect on proptosis (no different from placebo or IVGC in RCTs) or diplopia.Quality of life
Modest improvements in GO-QOL were demonstrated by one of the RCTs67 and an observational study101 both from the same center, and the latter including some data from the former. In the RCT at 52 weeks follow-up, 77% (10/13) of RTX-treated patients reported improved eye functioning QOL and 62% (8/13) improved appearance, compared with rates of 54% (7/13) and 46% (6/13), respectively, with IVGC.67
Dosing and route of administration
Among the two RCTs, 64% (18/28) patients were treated with a total dose of 2000 mg RTX, the remainder with 500 mg RTX.67,100 A post hoc analysis of three studies from a single center has examined different dosing regimens of RTX in 40 patients and found equivalent rates of disease inactivation and absence of relapse with all doses of RTX.102
However, the 100 mg dose failed to lead to disease inactivation or prevent progression to DON in 14% (2/14) patients, and higher doses of RTX were associated with better diplopia outcomes, so the 500 mg was deemed to be optimal.102 Patients in the two RCTs67,100 were premedicated before receiving RTX using acetaminophen/paracetamol, intravenous hydrocortisone (100 mg) or IVMP (100 mg), and antihistamines.67
Nonresponse and relapses after completion of treatment
Nonresponse compared with placebo was reported in one small (n = 11) RCT.100 Among studies that have reported responses totaling ∼150 patients, relapses have not been reported.101Safety
The rate of all AEs in the reported literature is 33–87%.98 Minor AEs ranged between 6% (for the 100 mg dose) and 75%.53 Serious AEs, mostly infusion reactions related to cytokine release with transient visual loss, but rarely fatal (described in patients with rheumatoid arthritis receiving long-term RTX 1000 mg every 6 months), are reported in 6–14% of cases. In a pooled analysis of the two RCTs, a total of 26 AEs occurred in 21 of 28 (75%) patients, including 1 case of vasculitis and 2 cases of transient vision loss due to cytokine release.53 Progressive multifocal leukoencephalopathy has been reported rarely in patients receiving RTX, generally for treatment of non-Hodgkin's lymphoma or other hematological malignancies.103Cost
The cost of a 500 mg course of RTX is 24–28 times that of IVMP (Table 7).Summary of evidence
There is contradictory evidence for the efficacy of RTX from two small single-center RCTs, but differences in baseline characteristics may explain the disparate results. Specifically, there was a shorter duration of TED in the study showing efficacy compared with the negative study (mean duration 4.5 months vs. 30 months).53 In addition, patients included in the negative study had higher CAS values, higher TRAb titers, and were more likely to be men and of older age, but less likely to be smokers than in the study showing benefit.53
The principal benefit is disease inactivation with no clinically significant effects on proptosis or diplopia. Modest effects on QOL have been reported in some reports. The response is durable at 1 year with a negligible relapse rate reported to date.53 Patients who have been previously treated with GCs and remain active with moderate-to-severe TED often respond to RTX.101 Doses between 100 and 2000 mg appear to be effective. On balance the evidence favors efficacy of RTX for disease inactivation (including previously GC-treated patients), with a low risk of relapse. Superiority to IVGC has been demonstrated in only one small RCT. The cost of RTX is significantly greater than that of IVMP.
Key Point 7.1.4.1: Evidence from RCTs is limited and divergent but suggests efficacy of RTX for inactivation of TED and prevention of relapses at >1 year, particularly in patients with TED of <9 months' duration.
Key Point 7.1.4.2: RTX therapy is acceptable in patients with active moderate-to-severe TED and prominent soft tissue involvement.
7.1.5. Mycophenolate
Mode of action
Mycophenolate exerts its immunomodulatory effects by inhibiting guanosine monophosphate synthesis, T and B cell proliferation, suppresses antibody production, and interferes with chemotaxis.104
Clinical experience
Mycophenolate has been used in a large number of patients, mostly for prevention of transplant rejection, and in patients with autoimmune diseases.105 The published experience in TED is limited to two RCTs,68,106 one nonrandomized trial107 and one retrospective report.108
Efficacy
Two RCTs have studied mycophenolate in patients with active moderate-to-severe TED. The first RCT was a single-center study and compared GC with mycophenolate mofetil, both administered for 24 weeks.106 The second RCT compared IVGC given for 12 weeks with IVGC plus mycophenolate sodium for 24 weeks.68 A third study was a retrospective audit with a highly heterogeneous population of 20 patients with limited efficacy data and will not be considered any further.108 Finally, a recent nonrandomized trial examined the use of mycophenolate mofetil plus oral prednisolone in 242 patients with moderate-to-severe TED.107Composite outcome
In the first RCT, the primary outcome was defined as improvement in ≥3 components of a composite, including improvement in CAS ≥2 or inactivation (CAS ≤3), improvement in soft tissue involvement by one grade in any of the following: eyelid swelling, eyelid erythema, conjunctival redness or conjunctival edema, reduction in proptosis ≥2 mm, improvement in eye movement (disappearance or reduction in severity of decreased eye movements), improvement in diplopia, or an increase in visual acuity ≥2/10.106 The primary outcome favored mycophenolate at 12 weeks, with 79% achieving the primary outcome versus 51% of those given IVGC, and at 24 weeks (91% vs. 68%).
The second RCT, which used the EUGOGO composite index (improvement defined as greater than or equal to two components among eyelid swelling, CAS, proptosis, lid width, diplopia, or eye muscle motility),19 found no differences in the composite index at 12 weeks between IVGC and IVGC plus mycophenolate sodium, and no differences in relapse rates at 24 and 36 weeks in the two groups.68 However, in a post hoc analysis, a significantly greater improvement was detected in mycophenolate-treated patients at 36 weeks, with 67% (49/73) of patients improving vs. 46% (31/68) patients improving with IVMP.68Activity
The first RCT found significant reductions in CAS within each group from baseline.106 Comparisons between groups showed no difference in mean CAS at 12 or 24 weeks, but the proportion of patients with disease inactivation, defined as CAS ≤3/10 at 24 weeks, favored mycophenolate mofetil, with inactivation occurring in 94% (69/80) of patients treated with this drug versus 69% (54/78) of those treated with GC. In the second RCT, CAS improved from baseline in both groups but there were no differences between groups.68Severity
The first RCT found a similar degree of improvement in proptosis in both groups at 24 weeks (mycophenolate mofetil −3.4 mm vs. GC −2.2 mm), but improvement occurred in a significantly higher percentage of mycophenolate mofetil-treated patients (69%, 55/80) than in those receiving GC (40%, 31/78).106 Diplopia improved in both groups at 24 weeks and the response was significantly better with mycophenolate than with GC (90%, 47/52 vs. 64%, 35/55). DON was not reported during follow-up in either group.
In the second RCT, proptosis and diplopia did not change from baseline in either group and there was no difference between groups, while DON occurred in both groups, including 9% (7/75) of patients receiving mycophenolate plus IVMP and 6% (4/72) of patients receiving IVMP alone.68 The nonrandomized study of 242 patients with moderate-to-severe TED noted improvement in proptosis, and diplopia in 83% and 94.2% of patients, respectively, at 12 months.107Quality of life
QOL was not assessed in the first RCT.106 In the second RCT, patients in both arms of the study noted slight improvement in QOL (<10 points improvement on the GO-QOL questionnaire), but there was no difference between groups.68
Dosing and route of administration
The first RCT compared GC in the form of IVMP 0.5 g on 3 consecutive days for 2 consecutive weeks followed by oral prednisone 60 mg daily for 8 weeks, and then tapering over the final 14 weeks (giving a cumulative dose of 6.7 g dose, for 24 weeks), with mycophenolate mofetil 500 mg twice daily for 24 weeks.106 The second RCT used IVGC 4.5 g cumulative dose for 12 weeks compared with IVGC (same regimen) plus mycophenolate sodium 360 mg twice daily for 24 weeks.68
Nonresponse and relapses after completion of treatment
The first RCT reported “reactivation” (without providing a definition) in 6.4% (5/78) GCs versus 0% (0/80) in the mycophenolate mofetil group.106 In the second RCT, relapses occurred in both groups, but between-group differences were not significant at 24 weeks [combination therapy 8% (4/53), IVGC monotherapy 11% (4/38)] or at 36 weeks [8.2% (6/73) versus IVGC 10.3% (7/68)].68Safety
In the first RCT, the rate of all AEs was significantly higher with GC, occurring in 28% (22/79) compared with mycophenolate mofetil occurring in 5% (4/80).106 The serious AE rate was 1.3% (1/79) for GC and 0% (0/80) for mycophenolate mofetil. In the second RCT, mild and moderate (grade 1–2) AEs also occurred in both groups including 47% (39/83) of patients treated with IVGC plus mycophenolate mofetil versus 36% (29/81) for those receiving GC alone, without statistically significant between-group differences. Serious AEs also occurred to a similar extent in both groups, including 16% (13/83) of patients receiving mycophenolate mofetil plus IVGC and 12% (10/81) of those given IVMP alone.68Cost
The cost of a course of mycophenolate mofetil as described in the two RCTs is between five and seven times that of a course of IVGC68,106 (Table 7).Summary of evidence
The first RCT demonstrated significant superiority of mycophenolate mofetil compared with IVGC in primary end points (composite outcome), as well as CAS, proptosis, diplopia, relapses, development of DON, and safety.106 Indeed, the response to mycophenolate mofetil in this population far exceeded that reported for any medical treatment for TED. Conversely, the second RCT was negative in terms of its primary objectives, although a significant difference in the composite outcome at 36 weeks (but not at 12 or 24 weeks) was observed in a post hoc analysis, of uncertain clinical significance.
Although there were differences between the two study populations within demographics such as age and geographical location, smoking history, the use of concurrent therapy, and actual dose and preparation of mycophenolate delivered, these disparate outcomes are not easily explicable, and the lack of additional data to help understand the discrepancies suggests a need for additional efficacy data, to better define the role of this drug in TED. Recently, combination therapy IVMP plus mycophenolate was recommended as first-line therapy for TED in the EUGOGO clinical practice guidelines,109 but the limited data and inconsistent findings to date were deemed by the TF to be not sufficiently convincing.
7.1.6. Tocilizumab
Mode of action
Interleukin-6 is expressed in orbital fibroblasts of patients with TED and seems to drive inflammation.2 TCZ is an interleukin-6 receptor blocker.
Clinical experience
TCZ has been used extensively for inflammatory arthritis.110 Reports on its use in TED in the published literature are confined to <100 patients mostly from a single center.84,111,112
EfficacyActivity
TCZ was shown to be effective in inactivating TED in all treated patients of a small open-label study involving 18 patients with CAS ≥4.111 A small RCT followed in 32 GC-resistant patients with moderate-to-severe TED and baseline CAS ≥4.112 The primary end point (improvement in CAS by ≥2 at 16 weeks) was achieved in significantly more treated patients than those receiving placebo (93%, 14/15 vs. 59%, 10/17); however, there was no difference between groups by week 40. A real-world report of 54 patient with GC-resistant TED treated with TCZ for 9 years from the same center as the original open label study cited inactivation in 74% of patients.84Severity
The open-label study showed reduction in proptosis by a mean 3.92 mm in patients with GC-resistant TED and resolution of diplopia in 54% (7/13) of patients.111 The real-world study reported proptosis reduction ≥2 mm from baseline in 78% (42/54) of patients, and improvement in diplopia in 68% (19/28) of patients.84). In the RCT, proptosis values in TCZ-treated patients were significantly lower than those in placebo-treated patients at 16 weeks by a median of 1.5 mm; however, no differences in proptosis were demonstrable at 40 weeks, and diplopia improved in only 7% (1/15) of patients treated with TCZ.
Despite modest improvement in individual parameters, an objective composite index improved significantly more in TCZ- than in placebo-treated patients at 16 weeks (73%, 11/15 vs. 29%, 5/17) and this was sustained at 40 weeks (67%, 10/15 vs. 18%, 3/17).112Quality of life
In the RCT, QOL (GO-QOL and SF-36) improved more in the TCZ group than in the placebo group at 16 weeks, but there were no differences at 40 weeks.112 The observational studies84,111 did not report on QOL.
Dosing and route of administration
The studies in TED patients have used intravenous TCZ 8 mg/kg or placebo on weeks 0, 4, 8, and 12.84,111,112 A subcutaneous preparation of TCZ is now available and requires further exploration in TED.113,114
Nonresponse and relapses after completion of treatment
In the RCT, the nonresponse rate based on the primary end point (improvement in CAS by ≥2) compared with baseline was 7% (1/15) at 16 weeks and 13% (2/15) at 40 weeks.112 The RCT did not include relapses in its analysis.112 Relapses were not observed in the open-label study.111 The real-world study reported relapses in 7.4% of patients.84Safety
AEs include risk of severe infections, hepatotoxicity, and anaphylaxis. The RCT reported a total of 58 AEs in the TCZ and 33 in the placebo-treated patients by 40 weeks and included 2 serious AEs (transaminase elevation, pyelonephritis) among the 15 TCZ-treated patients.112 The observational studies84,111 reported mild or moderate AEs such as fatigue, upper respiratory infection, cellulitis, neutropenia, and mild transaminase elevation, occurring in up to 48% of patients.84Cost
The cost of a course of TCZ is 60–85 times that of IVGC (Table 7).Summary of evidence
The impressive outcomes from the observational studies (especially on proptosis)84,111 have not been reproduced to the same degree by a single small RCT, although overall efficacy of TCZ was confirmed in GC-resistant patients with TED.112 An ongoing multicenter trial is testing intravenous TCZ efficacy in comparison with IVGCs and will further inform on the place of this drug in the routine management of TED (EudraCT Number: 2018-002790-22, ClinicalTrials.gov Identifier: NCT04876534).
Key Point 7.1.6.1: TCZ is an acceptable treatment for TED inactivation in GC-resistant patients with active moderate-to-severe disease.
7.1.7. Other agents
7.1.7.1. Other agents tested in TED patients and clinically available
Several additional agents have been tried in TED (e.g., atorvastatin, methotrexate, intravenous immunoglobulin (IVIG), azathioprine, cyclosporine, somatostatin analogues, and tumor necrosis factor (TNF) alpha inhibitors. Only a few have been studied in RCTs.
An RCT comparing atorvastatin 20 mg daily × 24 weeks plus IVMP (500 mg IV weekly × 6 weeks followed by 250 mg weekly × 6 weeks) with IVMP alone found significantly greater improvement in the EUGOGO composite index (51%, 21/41, vs. 28%, 11/39 patients), and relapses at 24 weeks were less likely in the atorvastatin plus IVMP arm (0/41 patients) versus the IVMP alone arm (15%, 6/39 patients).71 The GO-QOL improved significantly more in the combined therapy group (by 6.4 points) compared with that in the IVMP group.
Despite greater improvement in the composite index when atorvastatin was added to IVMP, there were no significant differences between groups in individual eye components such as CAS and diplopia, which improved in both groups, or proptosis, visual acuity, and eye aperture, which improved in neither group.71
IVIG appeared to have efficacy comparable with OGC in the one and only RCT,115 but because of the high cost, risk of transmission of infections and availability of other treatments, IVIG is not currently used in TED.
The roles of azathioprine (one RCT), cyclosporine (two RCTs) TNF alpha inhibitors, somatostatin analogues (four RCTs), and methotrexate are questionable as the evidence is either anecdotal or indicates lack of efficacy, or the side effect profile is unfavorable.65 Unfortunately, the evaluation of these agents has been done utilizing a multitude of outcomes along with different definitions for relapse rates after a successful outcome, thus precluding an easy comparison between these agents.
7.1.7.2. Other agents under investigation in TED patients but not clinically available
A recent study aimed at decreasing the half-life of IgG with a neonatal fragment crystallizable receptor inhibitor (IMVT-1401) was terminated early due to concerns about dyslipidemia (ClinicalTrials.gov Identifier: NCT03938545). Belimumab, an anti-B cell activating factor monoclonal antibody, was compared with IVGCs in a randomized trial (EudraCT Number: 2015-002127-26)116 with potentially promising results that have not been published at the time of this writing.
7.1.7.3. Other agents tested in GD patients with potential benefit in TED but not clinically available
Inferentially, a group of agents that have been tested as therapy for GD could ultimately prove beneficial for TED. Iscalimab blocks TSHR activation through the inhibition of intracellular activities leading to TRAb formation,117 and ATX-GD59 is intended to induce tolerance to TSHR.118 Both agents have been tested in small studies with encouraging results. A TSHR blocking monoclonal antibody (K1-70) (ClinicalTrials.gov Identifier: NCT02904330) is showing encouraging results in GD and also improvement in TED in the few patients studied who had both conditions.119
This was a phase 1 study and further investigation of this therapy is needed before a clear indication for TED can emerge. This and other planned studies with small molecule antagonists to the TSHR (S37a, ANTAG3) will possibly add to the armamentarium against TED in the future.
7.2. Radiotherapy for moderate-to-severe TED
RT has been used to treat TED for >70 years and may work by inhibiting or depleting lymphocytes and fibrocytes in the involved orbital tissue. The efficacy of RT for TED is variable in clinical studies to date, and interpretation is hampered by divergent inclusion criteria and outcome analyses.120 Proponents of RT cite a reduction in periocular inflammation in 60% of patients with active TED, a rate equivalent to OGC but less than that seen with IVGC.121 Data from two observational studies have shown a prolonged duration of effect from RT that may provide a GC-sparing effect, allowing an earlier tapering of OGC.122,123
RT has been compared with sham RT in three prospective studies. Two trials from the Netherlands randomized a total of 147 subjects with progressive TED and found the irradiated group ultimately had better ocular motility, manifested by improved excursions and less diplopia.124,125 Conversely, an American RCT comparing RT on one eye with sham therapy on the opposite side in 42 subjects with longer standing disease (median TED duration 1.3 years, range 0.2–16 years) found no benefit in a composite outcome of proptosis, lid retraction, and soft tissue index.126 The latter study supports the observation that RT is ineffective for late-stage or inactive disease.
Several studies have assessed the benefit of adding RT to GC therapy in TED. Two small RCTs with a combined total of 40 participants with active TED found greater response based on global severity scores in the combined RT plus OGC group than in the OGC control group.127,128 A retrospective Canadian study reviewed 351 patients with progressive TED who received either IVGC alone or IVGC combined with RT. At an average of 3.2 years follow-up, DON had developed in 17% of the IVGC group but in none of the combined therapy group, and the group with adjunctive RT also had a significantly greater improvement in ocular motility.77
Two additional retrospective analyses comparing IVGC with or without RT noted marginally increased benefit in the combined therapy group.129,130 However, a recent RCT from the United Kingdom (CIRTED Trial) found no gain from the addition of RT to OGC in subjects with active TED and moderately severe disease, in terms of a binary composite clinical outcome score or in terms of CAS.64 It is unclear whether the addition of oral or IVGCs amplifies the clinical response to RT.
The standard dosing protocol for early progressive disease since 1973 is 20 Gray (2000 Rads) divided over 10 days, delivered to the retrobulbar orbit through a lateral port, avoiding ocular or intracranial exposure.120 Two studies found equivalent efficacy when doses were reduced or divided into a greater number of fractions.131,132
Modern linear accelerator RT units have an improved safety record with retrospective series in TED showing no increased risk of cataracts,133 although a benign meningioma in the radiation field has been identified in a case report.134 Because of a theoretical lifetime risk of developing tumors, its use for TED is relatively contraindicated in people <35 years. RT may also increase the incidence of retinal vascular disease in patients with diabetes mellitus or hypertension.120 Orbital edema may increase during RT but can be controlled by concurrent GC.
Key Point 7.2.1: RT is a preferred treatment in patients with active moderate-to-severe TED whose principal feature is progressive diplopia.
Key Point 7.2.2: RT should be used cautiously in diabetic patients to avoid possible retinopathy. It is relatively contraindicated for those younger than 35 years of age to avoid a theoretical lifetime risk of tumors developing in the radiation field.
7.3. Surgical intervention for inactive moderate-to-severe TED
7.3.1. Surgical intervention overview
Elective surgery to correct proptosis, strabismus, eyelid malposition, and fat pockets can be initiated in inactive TED where clinical stability has been maintained and a euthyroid status achieved before surgery. Ocular motility should generally be stable for 4–6 months before strabismus surgery is performed. Surgical rehabilitation for TED is a staged approach, addressing proptosis first, then strabismus, and eyelid changes last. Not all patients require all procedures. QOL improvements often occur as a result of surgical rehabilitation for TED.135,136
Key Point 7.3.1.1: Surgery for moderate-to-severe TED should be performed by an orbital surgeon experienced with these procedures and their complications.
Key Point 7.3.1.2: Rehabilitative surgery for moderate-to-severe TED should only be performed when the disease is inactive and euthyroidism has been achieved and maintained.
7.3.2. Orbital decompression
Orbital decompression reduces intraorbital pressure and proptosis resulting from expanded orbital tissues by removal of bony walls, resection of orbital fat, or both. Indications include disfiguring proptosis, chronic orbital congestion, globe subluxation (Fig. 2f), and DON. The outcomes and complications for DON decompression surgery are covered in Section 8.3.
The most common indications are to restore appearance in proptosis and improve comfort in congestive orbitopathy and exposure keratopathy. In mild cases, intraconal orbital fat may be resected in fat-predominant disease, or the lateral wall drilled or partially removed. Greater reduction may be achieved by removing the bony medial wall and/or floor, opening the periorbital envelope, and displacing orbital fat and muscle into adjoining sinuses. Approximately 2 mm of proptosis reduction may be expected for each wall removed or 2 cm3 of fat excision.137,138
A rare indication is to relieve longstanding soft tissue congestion. Affected individuals have high CAS/VISA inflammatory scores but have had no recent progression and are nonresponsive to medical intervention. Improved venous drainage after expansion of the orbital compartment can result in dramatic improvement in orbital soft tissue changes and relieve orbital pain (Supplementary Fig. S2a, b).
Specific complications are associated with each wall decompressed. Deep lateral or medial wall surgery may cause a cerebrospinal fluid leak from dural injury,139 while oscillopsia (visual bobbing) may result from adhesions between the lateral rectus and temporalis muscles. Cheek numbness and inferior displacement of the globe may occur with floor decompression, while sinusitis and anesthesia of the upper jaw and nose may result from medial wall surgery. New-onset strabismus may develop in 7–34% of cases, depending on factors such as the technique of orbital decompression used and the size and restriction of enlarged extraocular muscle.140
This is less common in cases of fat-targeted disease, with one large series showing new diplopia persisting at 6 months after retro-orbital fat dissection in 8.6% of patients.141 A smaller fat-to-orbit ratio is associated with a lower likelihood of developing new diplopia postoperatively.142
Key Point 7.3.2.1: The specific surgical approach should be tailored to the indication (DON, proptosis), type of orbitopathy (muscle or fat predominant congestive disease), and desired reduction in proptosis.
7.3.3. Strabismus procedures
Strabismus with diplopia and/or a compensatory head turn to restore monocular gaze may develop from initial swelling and subsequent fibrosis of affected EOMs, or complicating orbital decompression surgery. While waiting for the diplopia to stabilize, binocular single vision in the primary or reading position may be obtained by using Fresnel adhesive prisms applied to a spectacle lens. In cases where prismatic correction is ineffective, diplopia can be avoided by occluding the worst affected eye with a foil, tape, or contact paper on the spectacle lens. Injection in the affected muscle with botulinum toxin is occasionally used as a temporary measure to correct diplopia.143
The goal of strabismus surgery is to restore or expand the field of binocular single vision (Supplementary Fig. S2e, f) and hence improve QOL.144 Once strabismus measurements have stabilized for at least 6 months, the restricted rectus muscles are typically recessed by releasing them from their insertion site and reinserting them by a variable amount further back in the globe, based on the desired correction, through a transconjunctival approach. Adjustable sutures may be used, which can be shifted after wakening the patients based on their feedback.145 Muscle tendons may be lengthened using donor tissue or hang-back sutures for large deviations.146
In severe strabismus, several surgical procedures on different muscles may be required and the field of binocular single vision may remain limited. After a large inferior rectus muscle recession, secondary lower lid retraction may develop. Patients deferring surgery or with smaller deviations may be helped with permanent prisms ground into the spectacle lenses.
Key Point 7.3.3.2: In patients with diplopia and inactive TED, binocular single vision in the primary position of gaze may be restored with strabismus surgery or permanent prisms ground into the spectacle lenses.
7.3.4. Eyelid procedures
Eyelid correction is performed in stages, usually addressing upper or lower retraction first, and concerns about appearance such as swelling or the adjacent glabellar folds second (Supplementary Fig. S2 g, h and i, j). In cases with significant proptosis, a preceding decompression surgery often results in a better reconstructive outcome from the lid surgery. Upper lid retraction may result from a fibrotic levator muscle or in compensation for a restricted inferior rectus muscle and is characterized by scleral show, lateral flare (retraction) (Fig. 2d), and lagophthalmos (Fig. 2a).
During the early progressive phase, upper lid retraction may temporarily respond to triamcinolone injection into the supratarsal subconjunctival space.147 The upper lid may be lowered by releasing the retractor muscle from an anterior or posterior approach.148 The retracted lower lid may be elevated with the use of autologous or allograft spacer materials.
Correction of upper lid fat prolapse in TED is achieved with a customized blepharoplasty addressing the excess of the preaponeurotic and sub-brow fat pads, and lacrimal gland prolapse. Botulinum toxin can be injected into the muscles between the brows to relax the vertical frown line.
Key Point 7.3.4.1: Eyelid retraction and fat prolapse are surgically corrected when TED is inactive and euthyroidism is achieved, and after decompression and strabismus surgery as indicated.
8. THERAPY FOR SIGHT-THREATENING TED
8.1. Intravenous glucocorticoids
DON may result from compression of the optic nerve by enlarged EOM at the apex of the orbit (Fig. 3a, b), or infrequently (<5%), due to stretch of the nerve because of proptosis. It is important to distinguish these two forms radiographically, as optic nerve stretch does not respond to medical treatments and requires surgical decompression to reduce proptosis.24
For many years, orbital decompression has been the standard treatment for DON but IVGCs have proven effective as well, and are now used first, to possibly avoid surgery.149 Although the optimal dose and schedule of GC are not established, the recommended use of large doses (0.5–1.0 g) of IVMP daily for 3 consecutive150 or alternate days,151 is based on the experience of treating patients with optic neuritis from other etiologies.152
The existing literature defines the response to IVGC rather broadly as “visual recovery,” but does not provide quantitative data on improvements in visual fields and color vision. IVGC has been reported to be effective in ∼40% of DON patients, generating improvements in visual acuity and avoiding subsequent orbital decompression.151,153 Therefore, IVGC should generally be considered as the preferred treatment with the purpose of avoiding or postponing surgery.151 The presence of optic disk swelling or atrophy at diagnosis are predictors of inadequate response to IVGC,153 but should not deter a trial of these drugs to assess efficacy in a particular patient.
Visual deterioration 2 weeks after initiating therapy is also predictive or poor response to IVGC. Although late surgical decompression can still provide benefit for DON, it may not allow complete restoration of normal visual function.154–156 Recent reports of effectiveness at treating DON by mycophenolate,108 TEP,157 and TCZ158 require confirmation in RCTs.
Key Point 8.1.1: Patients with DON require urgent treatment with IVGC therapy, with close monitoring of response and early (after 2 weeks) consideration for decompression surgery if baseline visual function is not restored and maintained with medical therapy.
8.2. Radiotherapy in DON
The role of combined RT and GC in prevention of DON in high-risk patients and in reducing the need for surgical decompression in patients with existing DON remains controversial. Evidence from three large retrospective studies indicates that this approach may reduce the incidence of DON in high-risk patients77 and may delay or obviate the need for decompression surgery in patients with established DON.159,160 A prospective study is currently underway by the International Thyroid Eye Disease Society (ITEDS) to confirm this preventive application (Clinical Trials.gov identifier: NCT02339142).
Most patients with DON or at high risk of DON (Table 2) have progressive diplopia or reduced ocular motility and so are already candidates for RT (Section 7.2, Key Point 5.2.1) and likely to benefit from such treatment.
Key Point 8.2.1: RT may be considered for preventing or as an adjunct to treating DON.
8.3. Orbital decompression for DON
Orbital decompression has been recommended for cases of recent-onset or progressive DON who respond incompletely or only transiently to immunosuppressive therapy.151 In most cases, apical compression of the optic nerve by swollen EOMs is relieved by decompression of the deep medial and inferior orbital wall through a transcaruncular or transnasal endoscopic approach. Visual improvement may be noted within days of the procedure, and even severe or longstanding visual loss may have partial or full visual recovery.24
Strabismus is more likely from these surgeries as the muscles are already inflamed.24 Complications include cerebrospinal fluid rhinorrhea or rarely an intracranial hemorrhage.161 Orbital decompression for the rare case of stretch optic neuropathy is usually designed to maximize reduction of proptosis by expansion into adjoining sinuses and fat excision.162 Patients who require orbital decompression for DON during the active progressive phase of TED may require adjunctive therapy with medical treatments or RT aiming to inactivate the disease (Table 5).
Occasionally vision loss may persist due to irreversible optic nerve atrophy despite combined medical and surgical therapy.163 Risk factors include advanced age, comorbidities such as diabetes mellitus, and delays to treatment. Poor response to a trial of IVGC and evidence of optic nerve atrophy on OCT predict a less favorable outcome. A postoperative CT scan can indicate whether additional surgical apical decompression is possible.
Key Point 8.3.1: In patients with compressive DON, orbital decompression of the deep medial wall and orbital floor should be considered to restore vision by reducing apical compression on the optic nerve.
9. OVERVIEW OF THE MANAGEMENT OF TED
Figure 5 shows an overview of the suggested management of TED. Despite great progress in recent decades, the management of TED remains a challenge (except in the mildest cases). Because of clinical disease heterogeneity and insufficient published evidence on this topic (i.e., scarcity of rigorous RCTs), robust recommendations regarding first-line and second-line treatments are challenging. An individualized approach to the management of TED, based on disease activity, severity, duration, trend across time, impact of the disease on daily living, treatment goals, patient age, and comorbidities, as well as the availability and relative costs of such therapies, is advised.
Treatment options during both the active phase (generally, immunomodulatory drugs) and the inactive phase (generally, corrective surgical procedures) should be carefully discussed with patients. Finally, regional and even local health care system differences impact the availability of current therapies, and these factors become critical in the individualization of care.
10. RESEARCH GAPS IN THE MANAGEMENT OF TED
Table 9 lists gaps in the understanding of TED and its management that the TF deemed to have importance as the focus of further clinical research.
Table 9.
Research Gaps in the Management of Thyroid Eye Disease
Identifying TED or those at risk for TED Are there reliable biomarkers to predict the development of TED in patients with newly diagnosed GD? Are there reliable biomarkers to assess TED activity more accurately than CAS? Is there a simple clinical screening tool to identify patients with early TED? Is there a simple and easy screening tool that patients with GD can use to self-diagnose TED early? Is race a risk factor for TED? What are the underlying mechanisms whereby radioactive iodine increases the risk of TED?Assessment of patients with TED How does vision, inflammation, strabismus, appearance compare with CAS for reproducibility and for predicting response to treatment? Are there more objective and reproducible methods than clinical examination to document the features of TED (e.g., photogrammetry)? How do we best utilize QOL measures (e.g., GO-QOL, TED QOL) to guide everyday clinical practice?Treatment of mild TED Is selenium useful in selenium sufficient areas? Is elevation of head of bed of any value in patients with TED?Treatment of moderate-to-severe TED How does TEP compare with IVGC therapy in head-to-head comparison studies? What is the durability of clinical response after TEP therapy? What is the optimal dosing and duration of TEP therapy? Is TEP therapy cost-effective at current prices? What is the effectiveness of TEP therapy for inactive and/or protracted TED (>12 months duration) What is the role of mycophenolate mofetil? Is there a role for thyrotropin receptor blocking agents in the management of TED? Is combined treatment of IVGC and RT more efficacious than IVGC alone? What is the efficacy and optimal dosing of RTX? What are the most relevant outcome measures in clinical trials for TED? What is the impact of medical therapies on subsequent surgical management? Is selenium helpful in moderate-to-mild TED? Is there a role for statins?Treatment of recurrent or refractory TED What are the most effective treatment choices for recurrent TED?Pathogenesis of TED What components of tobacco smoke contribute to TED? How effective is smoking cessation? What is and how do we separate “congestive” TED from active TED?Health care models for the management of TED What is the most clinically effective and cost-effective specialty TED care model? What is the impact of current drug costs, affordability, and limited global availability on health disparities in TED?Ophthalmology-specific research What is the role of chin-up positioned eye assessment in TED (to eliminate gaze-dependent ocular hypertension and optic neuropathy in restrictive strabismus)? Is the Gorman diplopia score an optimal metric of ocular motility impairment in routine clinical practice and in clinical trials? What is the role of RT/GC vs. GC alone in treating cases of established DON and allowing avoidance of surgery?
Open in a new tab
GD, Graves' disease; QOL, quality of life.
Add a subtitle here
© 2025
























