Less is more for health and happiness
- …


Less is more for health and happiness
- …


Steps to reduce morbidity and improve your quality of life
Advices for your care-givers
Managing TED effectively involves a structured, stepwise approach that takes into account the disease’s activity and severity, as outlined by the European Group on Graves’ Orbitopathy (EUGOGO) guidelines, which are widely adopted in the UK and Europe.
Here’s a brief overview of the current management approach:
- Achievement of Euthyroidism: The first and essential step is to ensure the patient’s thyroid function is well controlled (euthyroid), as this underpins overall management and helps reduce disease progression.
- Assessment of Disease Activity and Severity: Using tools like the Clinical Activity Score (CAS) and EUGOGO’s severity classification (mild, moderate-to-severe, sight-threatening), clinicians tailor treatment strategies accordingly.
- Immunosuppression:Glucocorticoids (GCs), especially intravenous methylprednisolone, remain first-line treatment during the active inflammatory phase. They work by reducing inflammation through suppressing pro-inflammatory cytokines and immune cell recruitment. Early initiation during the active phase (on Rundle’s curve) yields the best results.
- Other Therapeutic Approaches: For moderate-to-severe active TED, newer therapies and a combination of treatments aim to reduce inflammation, reverse proptosis, and improve eye movement. Surgical options, including orbital decompression, eyelid surgery, and strabismus correction, are considered mainly for residual dysfunction and disfigurement once the disease has stabilized.
- Multidisciplinary Care: Optimal management involves a team approach, including endocrinologists and ophthalmologists, to address both thyroid and eye issues comprehensively.
- Patient-reported Outcomes and Monitoring: Tools like the Graves’ Ophthalmopathy Quality of Life questionnaire (GOQOL) and objective composite scores help monitor treatment response and guide further management.
This systematic approach allows for personalized care, improving both clinical outcomes and quality of life for patients living with TED.
- Measure serum TSH (Thyroid-stimulating hormone) receptor antibodies [ in general, and/or as functional assyas, especially thyroid stimulating antibodies (TSAbs) ]
Screen all patients with GD for TED at each visit
- Use specific tools to detect symptoms and signs of TED
- Analyze risk factors for development or progression of TED at first visit; consider use of predictive scoring
Alert all patients with GD to the risk of TED
- Use educational materials and GO early warning card
Prevent TED development or progression
- Refer for smoking cessation
- Achieve and maintain stable biochemical euthyroidism quickly
- Avoid radioactive iodine (RAI) in active, moderate-to-severe TED
- Use steroid prophylaxis following RAI in active TED
- Avoid hypothyroidism after RAI
- 6-month couse of selenium supplementation in mild TED
Refer to a specialist clinic early
- If possible, refer patients with moderate to severe and sight-threatening TED early to specialists with specific TED expertise
Refer to a specialist clinic early
- If possible, refer patients with moderate to severe and sight-threatening TED early to specialists with specific TED expertise
Systemic Medications
Systemic medications target the underlying inflammatory issues caused primarily by thyroid and thyroid hormone disfunction. There are number of medications available to thyroid eye disease patients, each of which require careful monitoring and adjustment based on individual response and potential side effects.
Corticosteroids
- Oral prednisone for acute inflammation
- IV methylprednisolone for severe cases
- Typically used for 3-6 months with careful monitoring
- Must be tapered gradually to prevent rebound
In some cases, acute swelling causing double vision or loss of vision may be treated for a limited time with oral prednisone. However, prednisone given for more than a few weeks at the dosages required to suppress the autoimmune inflammation often causes bothersome and dangerous side-effects that may become severe.
In patients who respond to prednisone, a short course of intravenous (IV) steroids (methylprednisolone) may provide symptomatic improvement with fewer side effects than oral prednisone; this is referred to as an IV steroid pulse.

Teprotumumab (Tepezza®)
A newly FDA-approved drug, teprotumumab (Tepezza®), has been shown to be effective in the majority of patients with active thyroid eye disease. (Patients who experience eye redness, pain with eye movement, worsening proptosis, and/or worsening diplopia.) Teprotumumab can be expensive, and requires insurance pre-authorization, but is a very good option for some patients.
- Eight infusions over 24-week period
- Specifically targets eye muscle and orbital fat inflammation
- Can reduce proptosis and double vision
- Most effective during active inflammatory phase

Selenium Supplementation
Selenium supplementation is a supportive treatment for thyroid conditions. A trace mineral, selenium has shown promise in helping to regulate thyroid hormones and improve immune function. A daily recommended dose of selenium may help reduce inflammation and improve mild eye symptoms associated with TED. Its antioxidant properties appear to help protect orbital tissues and may slow disease progression.
- May help mild cases
- Recommended dose of 100-200 mcg daily
- Most beneficial in the early, active phase
Rituximab
A monoclonal antibody targeting CD20, rituximab acts by depleting the body’s population of autoreactive B cells.
Tocilizumab
Tocilizumab is an IL-6 monoclonal antibody used to treat active TED. A proinflammatory cytokine, IL-6 is found in high concentrations in patients with TED.
Emerging therapies
As our understanding of the pathophysiology underlying TED advances, new molecular targets are identified.
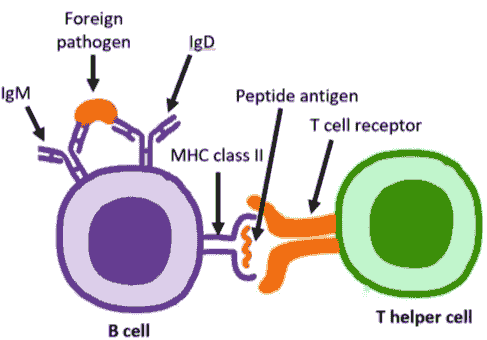
WP1302
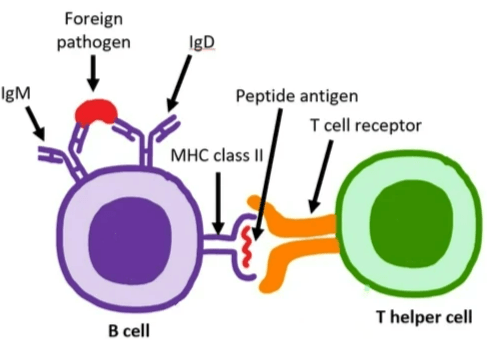
WP1302 is an antigen-specific immunotherapy based on CD4+ T-cell epitopes from TSHR. This antigen-specific immunotherapy is designed to functionally deprive B cells of the T-cell help required for production of stimulating autoantibodies through tolerance induction in TSHR-specific T cells.
WP1302 binds to major histocompatibility complex (MHC) class II molecules in the same conformation as the naturally processed T-cell epitope to engage disease related T cells. It is highly soluble and immune tolerogenic, different from insoluble peptides which are immunogenic. WP1302 mimicking naturally processed CD4+ T-cell epitopes is defined as antigen processing independent epitopes, or apitopes. Administration of apitopes induces type 1 regulatory (Tr1)-like T cells with immunosuppressive properties; such an approach has the potential for treatment of GD and prevention of TED.
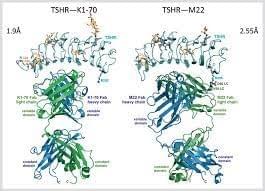
K1-70
K1-70 is a human monoclonal antibody targeting TSHR. K1‐70™ binds specifically to the TSHR with high affinity and prevents receptor binding by TSH and by patient TRAb thus blocking the stimulating actions of these ligands. In an open label phase I study on patients stable on ATD treatment, progression from hyperthyroidism or euthyroidism (at the time of dosing) to the hypothyroid state occurred in 12/18 (67%) of subjects, while in higher dose cohorts 9/9 subjects (100%) experienced hypothyroidism irrespective of their thyroid status at dosing.
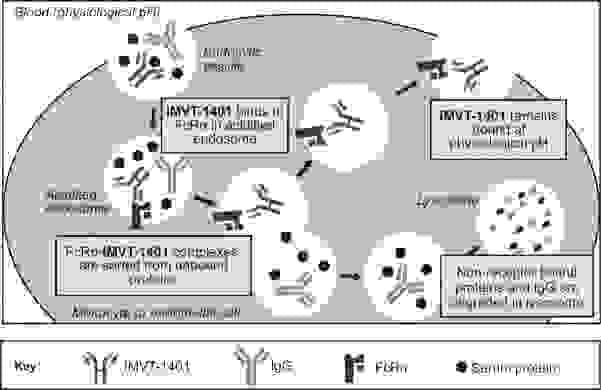
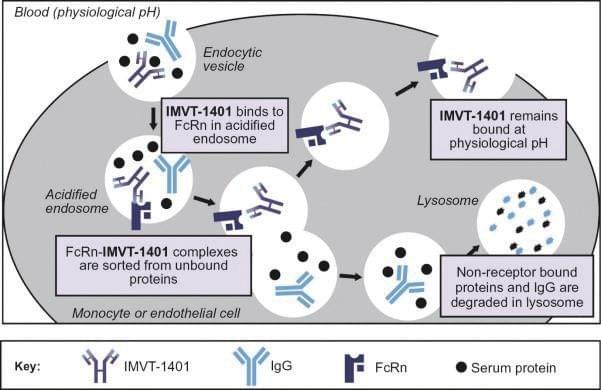
Batoclimab
Batoclimab is a monoclonal antibody targeting the neonatal Fc receptor (FcRn) that has been proposed for the treatment of several autoimmune diseases, including TED. FcRn transports immunoglobulin G (IgG) and prevents their lysosomal degradation. Batoclimab blocks FcRnmediated recycling of IgG and increases the catabolism of IgG in the body.
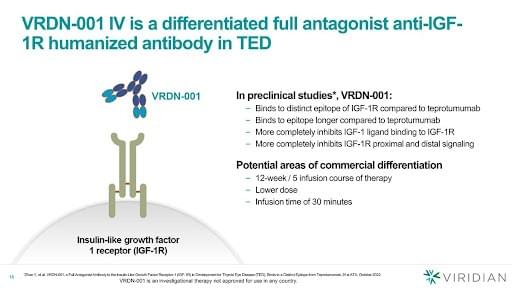
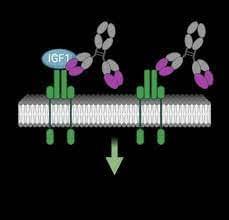
VRDN-001, VRDN-002 and VRDN-003
VRDN-001, a monoclonal antibody against the IGF-1R, is under investigation as a treatment option for TED. VRDN-002 is an anti-IGF-1R antibody compound with a modified Fc region engineered to extend the half-life of the drug. Additionally, VRDN-003 is another half-life-extended version of VRDN-001.
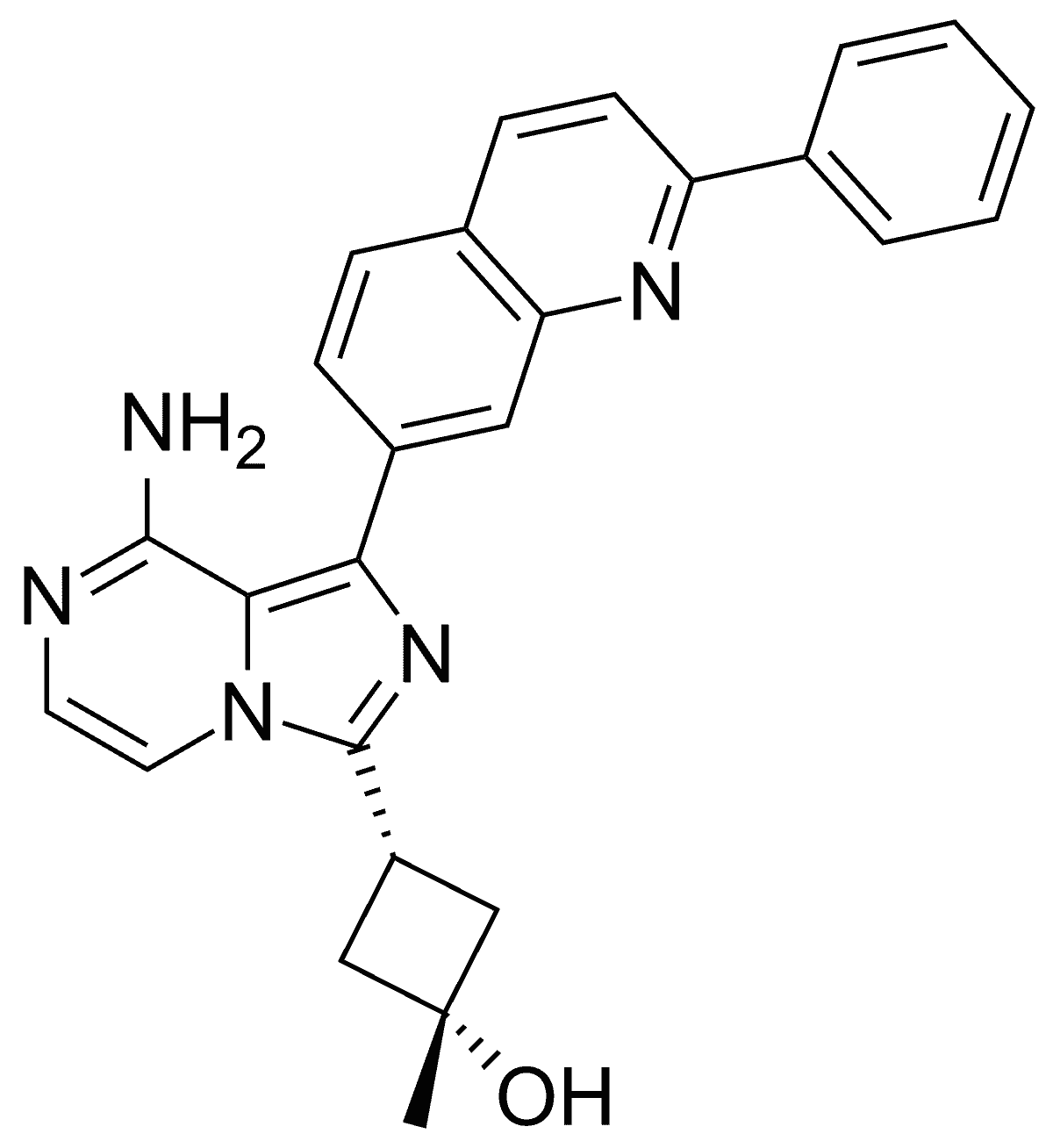
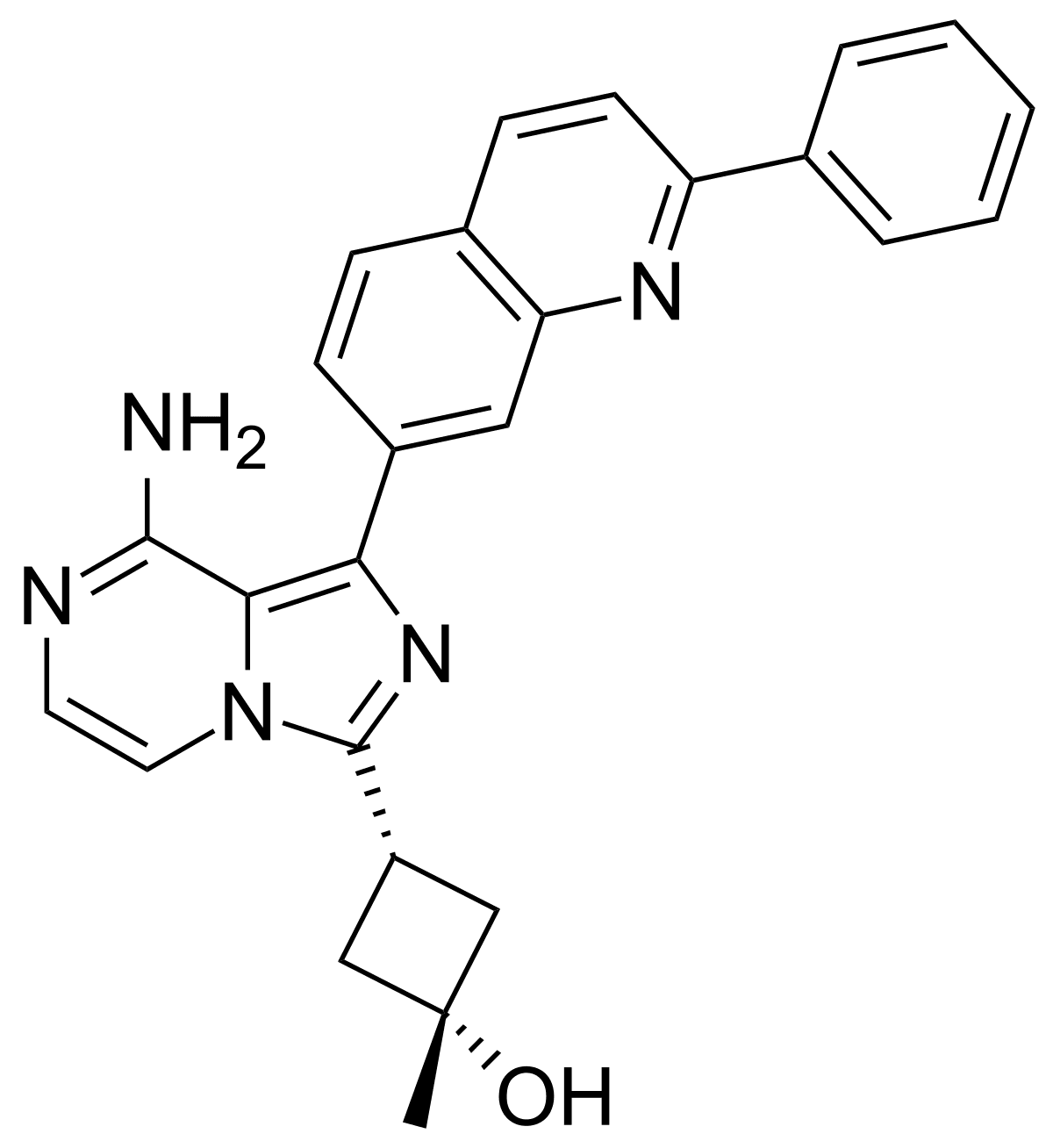
Linsitinib
Linsitinib is a small-molecule inhibitor of IGF-1R. In an experimental mouse model of TED, it was found to prevent autoimmune hyperthyroidism in the early stage of the disease and reduce the infiltration of orbital tissues by T cells and macrophages.
Lonigutamab
Lonigutamab is another anti-IGF-1R monoclonal antibody designed to have a strong binding affinity to its target, thereby potentially enabling a small-volume SC injection.
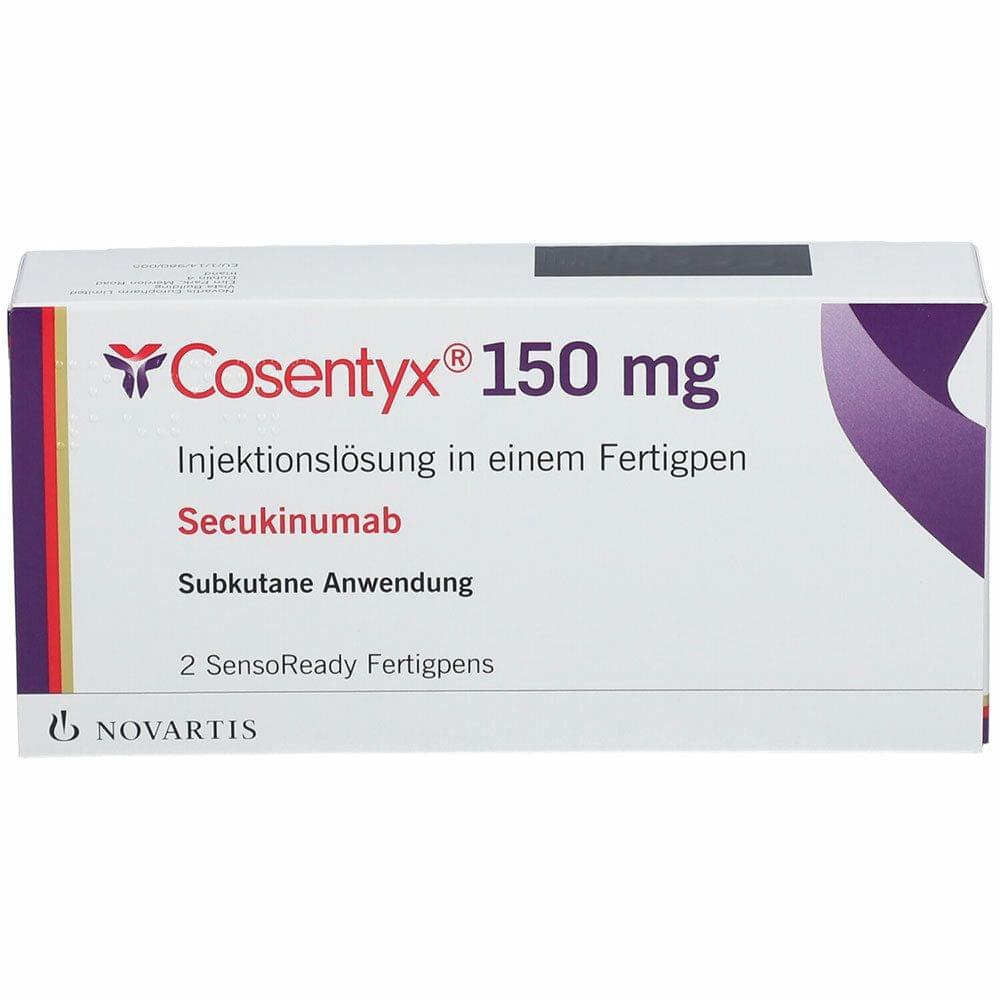
Secukinumab
Secukinumab is a human monoclonal anti-IL-17A antibody already approved for the treatment of psoriatic arthritis, ankylosing spondylitis and severe plaque psoriasis
© 2025